The concentration of acetylcholine (a neurotransmitter) in a sample can be determined from the pH changes that accompany its hydrolysis. When the sample is incubated with the enzyme acetylcholinesterase, acetylcholine is converted to choline and acetic acid, which dissociates to yield acetate and a hydrogen ion. CНз CHз Нао — сHz— CH "N— снз но-сн— сH,-"N-CH; + CH;— с-о+ н* CH3-C-0- сHз CHз Choline Acetylcholine Acetate In a typical analysis, 11 mL of an aqueous solution containing an unknown amount of acetylcholine had a pH of 7.70. When incubated with acetylcholinesterase, the pH of the solution decreased to 6.89. Assuming there was no buffer in the assay mixture, determine the number of moles of acetylcholine in the 11 mL sample.
The concentration of acetylcholine (a neurotransmitter) in a sample can be determined from the pH changes that accompany its hydrolysis. When the sample is incubated with the enzyme acetylcholinesterase, acetylcholine is converted to choline and acetic acid, which dissociates to yield acetate and a hydrogen ion. CНз CHз Нао — сHz— CH "N— снз но-сн— сH,-"N-CH; + CH;— с-о+ н* CH3-C-0- сHз CHз Choline Acetylcholine Acetate In a typical analysis, 11 mL of an aqueous solution containing an unknown amount of acetylcholine had a pH of 7.70. When incubated with acetylcholinesterase, the pH of the solution decreased to 6.89. Assuming there was no buffer in the assay mixture, determine the number of moles of acetylcholine in the 11 mL sample.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 116IL: Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO2H)...
Related questions
Question
100%
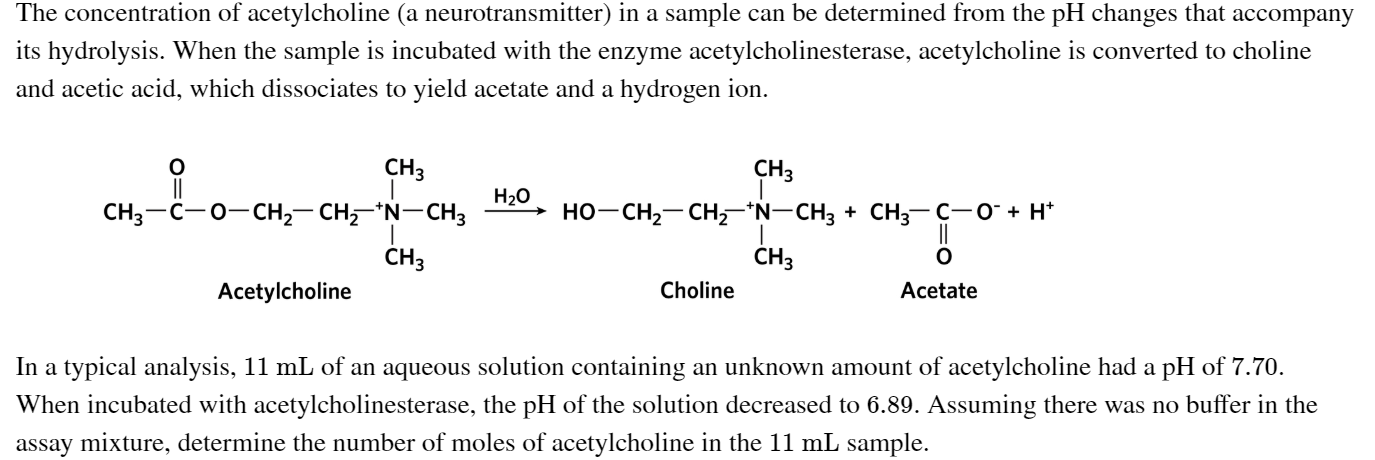
Transcribed Image Text:The concentration of acetylcholine (a neurotransmitter) in a sample can be determined from the pH changes that accompany
its hydrolysis. When the sample is incubated with the enzyme acetylcholinesterase, acetylcholine is converted to choline
and acetic acid, which dissociates to yield acetate and a hydrogen ion.
CНз
CHз
Нао
— сHz— CH "N— снз
но-сн— сH,-"N-CH; + CH;— с-о+ н*
CH3-C-0-
сHз
CHз
Choline
Acetylcholine
Acetate
In a typical analysis, 11 mL of an aqueous solution containing an unknown amount of acetylcholine had a pH of 7.70.
When incubated with acetylcholinesterase, the pH of the solution decreased to 6.89. Assuming there was no buffer in the
assay mixture, determine the number of moles of acetylcholine in the 11 mL sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning