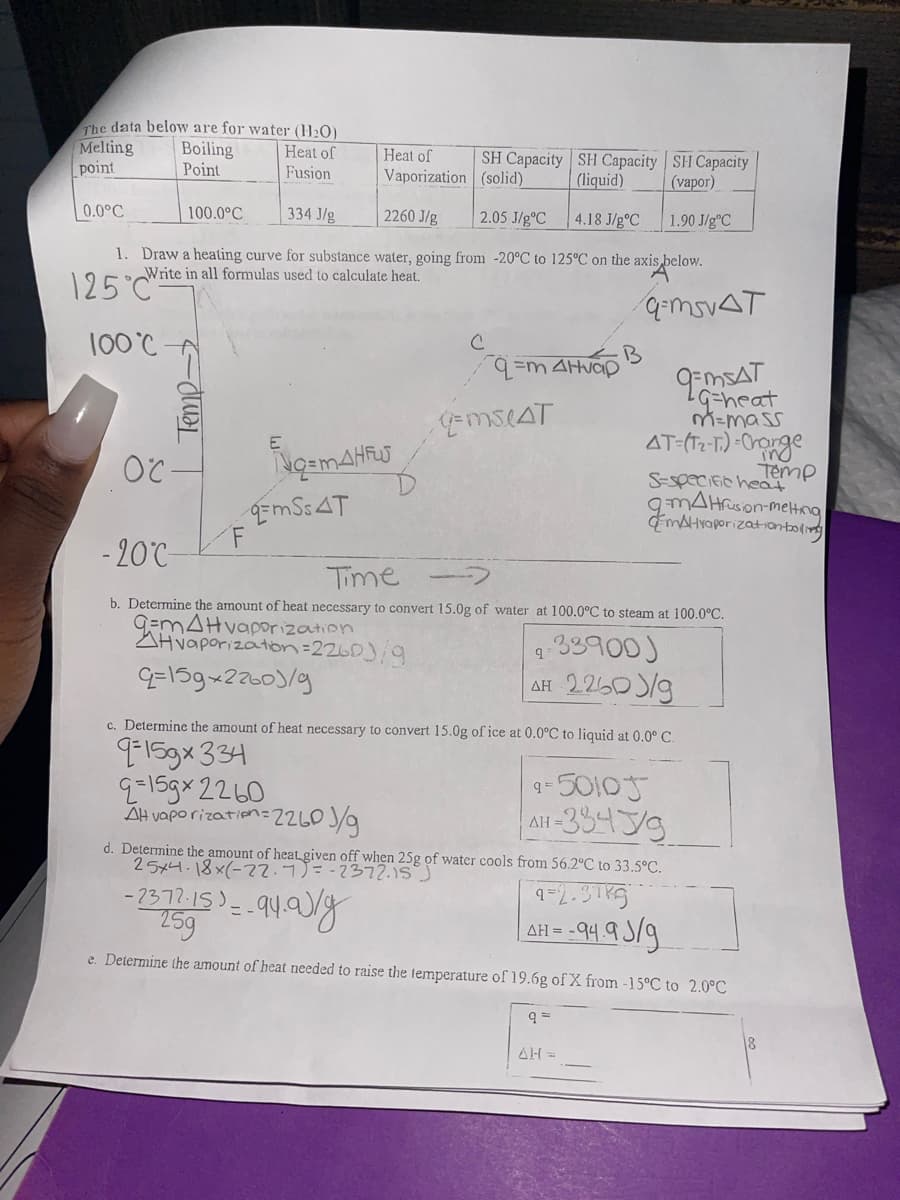
The data below are for water (H₂O) Melting Boiling Heat of point Point Fusion Heat of Vaporization SH Capacity (liquid) SH Capacity (vapor) 2260 J/g 4.18 J/g °C 1.90 J/gºC 1. Draw a heating curve for substance water, going from -20°C to 125°C on the axis below. Write in all formulas used to calculate heat. 25°C 100°C- 0.0°C 100.0°C Temp- OC- F E 334 J/g Nq=MAHFUS EmSSAT с SH Capacity (solid) 2.05 J/gºC q=m4Hvap q=mSLAT B q=mSVAT OMSAT Gheat m=mas AT (T2-T)-Char ir Te S-specific hea 9mAHfusion- mHvaporization -20°C- Time b. Determine the amount of heat necessary to convert 15.0g of water at 100.0°C to steam at 100.0°C. G=m4Hvaporization Hvaporization=2260)/9 9=159×22601/9 9-33900) AH 2260/9 AH c. Determine the amount of heat necessary to convert 15.0g of ice at 0.0°C to liquid at 0.0° C. q-159x 334 9=159x2260 4-50105 All-33439 AH vaporization=2260 J/g d. Determine the amount of heat given off when 25g of water cools from 56.2°C to 33.5°C. 25x4-18x(-22.7) = -2372.15) 9=2.315 -2372.15)=-94.9)/8 25g e. Determine the amount of heat needed to raise the temperature of 19.6g of X from -15°C to 2.0°C ΔΗ AH = -94.93/g
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Step by step
Solved in 8 steps with 1 images









