The density of a substance can change with temperature. The graph displays the density of water from -130 °C to 100 °C. Examine the graph and answer the questions. Water undergoes a large change in density at 0 °C as it freezes to form ice. Calculate the percent change in density that occurs when liquid water freezes to ice at 0 °C. final value - initial value (Hint: % change initial value × 100%) The Density of Water as a Function of Temperature 1,010 1,000 990 980 970 960 950 940 930 920 910 -150 -100 -50 50 100 Density (kg/m)
The density of a substance can change with temperature. The graph displays the density of water from -130 °C to 100 °C. Examine the graph and answer the questions. Water undergoes a large change in density at 0 °C as it freezes to form ice. Calculate the percent change in density that occurs when liquid water freezes to ice at 0 °C. final value - initial value (Hint: % change initial value × 100%) The Density of Water as a Function of Temperature 1,010 1,000 990 980 970 960 950 940 930 920 910 -150 -100 -50 50 100 Density (kg/m)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.89PAE: 1.89 Imagine that you place a cork measuring 1.30cm4.50cm3.00cm in a pan of water. On top of this...
Related questions
Question
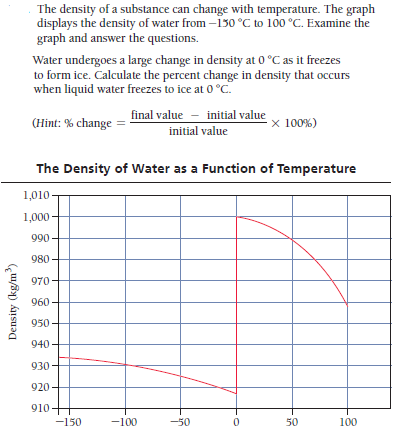
Transcribed Image Text:The density of a substance can change with temperature. The graph
displays the density of water from -130 °C to 100 °C. Examine the
graph and answer the questions.
Water undergoes a large change in density at 0 °C as it freezes
to form ice. Calculate the percent change in density that occurs
when liquid water freezes to ice at 0 °C.
final value - initial value
(Hint: % change
initial value
× 100%)
The Density of Water as a Function of Temperature
1,010
1,000
990
980
970
960
950
940
930
920
910
-150
-100
-50
50
100
Density (kg/m)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning