The energy of the incoming photon, Eotal, is conserved in photoelectron spectroscopy. Therefore, the energy of the incoming photon is equal to the , plus the , so The energy of the incoming photon can also be expressed as , and the kinetic energy of the outgoing electron can be expressed as . The energy required to eject the electron corresponds to the . Substituting these expressions into the original expression for Ewtal produces the desired relationship between the speed of the ejected electron and the frequency of the incoming radiation,
The energy of the incoming photon, Eotal, is conserved in photoelectron spectroscopy. Therefore, the energy of the incoming photon is equal to the , plus the , so The energy of the incoming photon can also be expressed as , and the kinetic energy of the outgoing electron can be expressed as . The energy required to eject the electron corresponds to the . Substituting these expressions into the original expression for Ewtal produces the desired relationship between the speed of the ejected electron and the frequency of the incoming radiation,
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.103PAE: 6.103 Atomic absorption spectroscopy is based on the atomic spectra of the elements being studied....
Related questions
Question
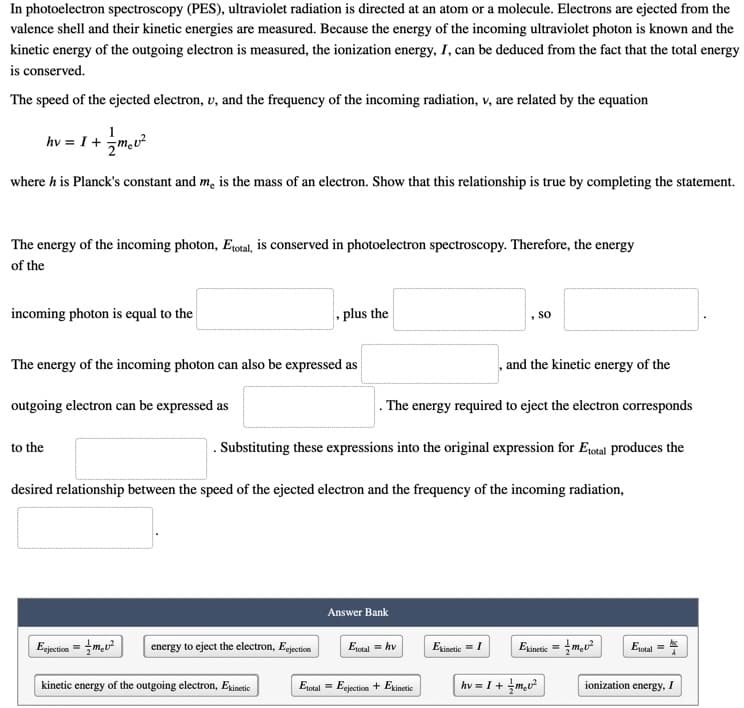
Transcribed Image Text:In photoelectron spectroscopy (PES), ultraviolet radiation is directed at an atom or a molecule. Electrons are ejected from the
valence shell and their kinetic energies are measured. Because the energy of the incoming ultraviolet photon is known and the
kinetic energy of the outgoing electron is measured, the ionization energy, I, can be deduced from the fact that the total energy
is conserved.
The speed of the ejected electron, v, and the frequency of the incoming radiation, v, are related by the equation
hv = I + m,u?
where h is Planck's constant and m, is the mass of an electron. Show that this relationship is true by completing the statement.
The energy of the incoming photon, Eotal, is conserved in photoelectron spectroscopy. Therefore, the energy
of the
incoming photon is equal to the
plus the
, so
The energy of the incoming photon can also be expressed as
and the kinetic energy of the
outgoing electron can be expressed as
. The energy required to eject the electron corresponds
to the
. Substituting these expressions into the original expression for E1otal produces the
desired relationship between the speed of the ejected electron and the frequency of the incoming radiation,
Answer Bank
Egjecticn = mu?
energy to eject the electron, Eejection
Eotal = hv
Exinetic =I
Exinetic = m,u?
E1otal
kinetic energy of the outgoing electron, Exinetic
Eotal = Eejection + Exinetic
hv = I+ +m,u²
jonization energy, I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning