The equilibrium constant for the proton transfer reaction of acetic acid 1.8 × 10–5 . Calculate the equilibrium concentration of acetic acid and acetate anions in a 0.050 M solution of the acid.
The equilibrium constant for the proton transfer reaction of acetic acid 1.8 × 10–5 . Calculate the equilibrium concentration of acetic acid and acetate anions in a 0.050 M solution of the acid.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 7P
Related questions
Question
The equilibrium constant for the proton transfer reaction of acetic acid 1.8 × 10–5 . Calculate the equilibrium concentration of acetic acid and acetate anions in a 0.050 M solution of the acid.
Expert Solution
Step 1
For the given reaction, the equation for equilibrium constant can be written as below.
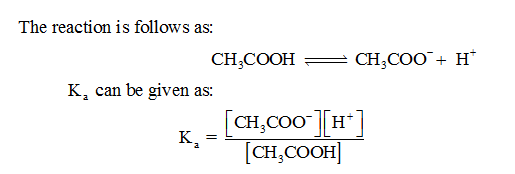
Step 2
Initial amount of acetic acid is given as 0.050 M. An ICE table is constructed to determine the concentration equilibrium.
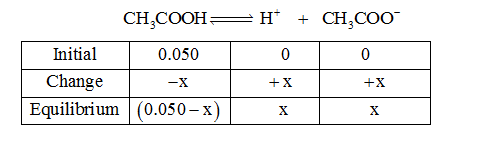
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning