The Eu(III) standards adled to the groundwater sample contained 0.160 ng/ml. (ppb) of Eu(llI). Calculate the concentration of Eu(lII) in the groundwater sample. Eu(III) concentration: ng/ml. The Th(III) standards adkled to the groundwater sample contained 16.0 ng/ml. (ppb) of Tb(III). Calculate the concentration of Th(III) in the groundwater sample. Th(III) concentration: ng/ml.
The Eu(III) standards adled to the groundwater sample contained 0.160 ng/ml. (ppb) of Eu(llI). Calculate the concentration of Eu(lII) in the groundwater sample. Eu(III) concentration: ng/ml. The Th(III) standards adkled to the groundwater sample contained 16.0 ng/ml. (ppb) of Tb(III). Calculate the concentration of Th(III) in the groundwater sample. Th(III) concentration: ng/ml.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.31QAP
Related questions
Question
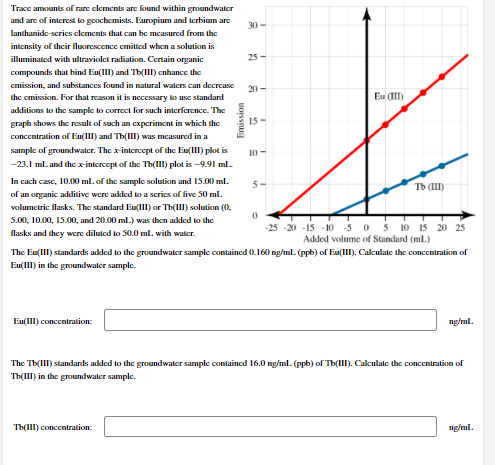
Transcribed Image Text:Trace amounts of rare clements are found within groundwater
and are of interest to goochemists. Earopium and terbium are
30 -
lanthanide-scries clements that can be measured from the
intensity of their fluorescence emittcd when a solution is
illuminated with ultraviolet radiation. Certain organic
25 -
compounds that bind Eu(III) and Tb(IIl) enhance the
cmission, and substances found in natural waters can decrease
20-
the cmission. For that reason it is necessary to use standard
Eu (II)
additions to the sample to correct for such interference. The
graph shows the result of such an cxperiment in which the
15-
concentration of Eu(III) and Tb(III) was mcasured in a
sample of groundwatler. The x-intercept of the Eu(III) plot is
10-
-23.1 ml. and the x-intercept of the Th(III) plot is -9.91 ml.
In cach case, 10.00 ml. of the sample solution and 15.00 ml.
5-
Tb (III)
of an organic additive were added to a scries of live 50 ml.
volumctric flasks. The standard Eu(lI) or Th(III) solution (0,
5.00, 10.00, 15.00, and 20.00 ml.) was then added to the
-25 -20 -15 -10 50 5 10 15 20 25
Added volume of Standard (mlL)
flasks and they were diluted to 50.0 ml. with water.
The Eu(III) standards added to the groundwater sample contained 0.160 ng/ml. (ppb) of Eu(III). Calculate the concentration of
Eu(III) in the groundwater sample.
Eu(III) concentration:
ng/ml.
The Tb(III) standards added to the groundwater sample contained 16.0 ng/ml. (ppb) of Tb(III). Calculate the concentration of
Th(III) in the groundwater sample.
Th(III) concentration:
ng/ml.
Emission
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning