The features of polar bonds can be illustrated by considering HF. The general form of the molecular orbital is y = GHY + GỰF, where y, is an H1s orbital and ựe is an F2p, orbital (with z along the internuclear axis, the convention for linear mol- ecules). The relevant data are leV Egglev H 13.6 0.75 F 17.4 3.40 Calculate the energies and coefficients of the bonding and antibonding orbitals made from this combination of atomic orbitals in HF.
The features of polar bonds can be illustrated by considering HF. The general form of the molecular orbital is y = GHY + GỰF, where y, is an H1s orbital and ựe is an F2p, orbital (with z along the internuclear axis, the convention for linear mol- ecules). The relevant data are leV Egglev H 13.6 0.75 F 17.4 3.40 Calculate the energies and coefficients of the bonding and antibonding orbitals made from this combination of atomic orbitals in HF.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter17: Conjugation And Molecular Orbital (mo) Theory
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
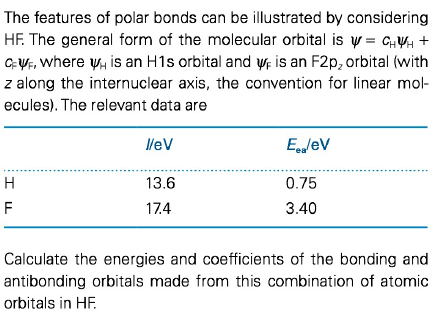
Transcribed Image Text:The features of polar bonds can be illustrated by considering
HF. The general form of the molecular orbital is y = GHY +
GỰF, where y, is an H1s orbital and ựe is an F2p, orbital (with
z along the internuclear axis, the convention for linear mol-
ecules). The relevant data are
leV
Egglev
H
13.6
0.75
F
17.4
3.40
Calculate the energies and coefficients of the bonding and
antibonding orbitals made from this combination of atomic
orbitals in HF.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning