The figure shows a two-dimensional gel of a mixture of proteins A, B, and C. The vertical axis represents SDS-PAGE separation, run from top to bottom. The horizontal axis represents isoelectric focusing with a pH gradient that runs from left(high pH) to right (low pH). В A Proteins A, B, and C have isoelectric points of 8.2, 6.7, and 5.2, respectively. С Place proteins A, B, and C in order of increasing elution volume on a size exclusion column. Assume they are high pH low isoelectric focusing monomers under the conditions of separation. pH SDS-PAGE Place proteins A, B, and C in order of probable increasing elution volume in cation exchange chromatography during an increasing salt gradient run at pH 5.
The figure shows a two-dimensional gel of a mixture of proteins A, B, and C. The vertical axis represents SDS-PAGE separation, run from top to bottom. The horizontal axis represents isoelectric focusing with a pH gradient that runs from left(high pH) to right (low pH). В A Proteins A, B, and C have isoelectric points of 8.2, 6.7, and 5.2, respectively. С Place proteins A, B, and C in order of increasing elution volume on a size exclusion column. Assume they are high pH low isoelectric focusing monomers under the conditions of separation. pH SDS-PAGE Place proteins A, B, and C in order of probable increasing elution volume in cation exchange chromatography during an increasing salt gradient run at pH 5.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter22: Proteins
Section: Chapter Questions
Problem 22.65P: 22-65 (a) What is the difference in the quaternary structure between fetal hemoglobin and adult...
Related questions
Question
Proteins A, B, and C have isoelctric points of 8.2, 6.7, and 5.2 respectively.
Place proteins A, B, and C in order of increasing volume on a size exclusion column.
Then place the proteins in order of probable increasing elution volume in cation exchange chromatography during an increasing salt graditend run at pH 5.
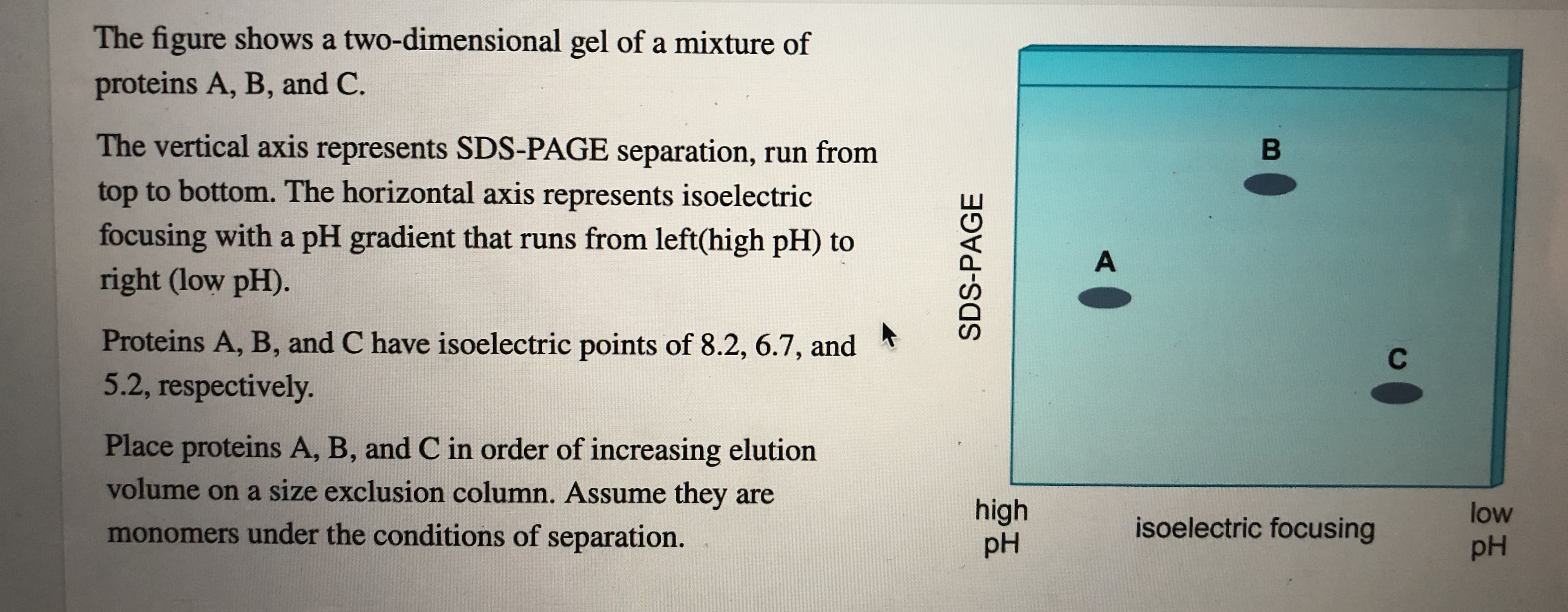
Transcribed Image Text:The figure shows a two-dimensional gel of a mixture of
proteins A, B, and C.
The vertical axis represents SDS-PAGE separation, run from
top to bottom. The horizontal axis represents isoelectric
focusing with a pH gradient that runs from left(high pH) to
right (low pH).
В
A
Proteins A, B, and C have isoelectric points of 8.2, 6.7, and
5.2, respectively.
С
Place proteins A, B, and C in order of increasing elution
volume on a size exclusion column. Assume they are
high
pH
low
isoelectric focusing
monomers under the conditions of separation.
pH
SDS-PAGE

Transcribed Image Text:Place proteins A, B, and C in order of probable increasing
elution volume in cation exchange chromatography during
an increasing salt gradient run at pH 5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning