The following diagram is a shell representation of an atom. When the atom is exposed to light, the electron absorbs light and undergoes excitation from ground state to excited state as shown in the diagram. ground etato eKcked stete
The following diagram is a shell representation of an atom. When the atom is exposed to light, the electron absorbs light and undergoes excitation from ground state to excited state as shown in the diagram. ground etato eKcked stete
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section11.2: The Hydrogen Atom
Problem 3RQ
Related questions
Question
4- Please I want answer with steps and clear typing or hand writing. Many thanks
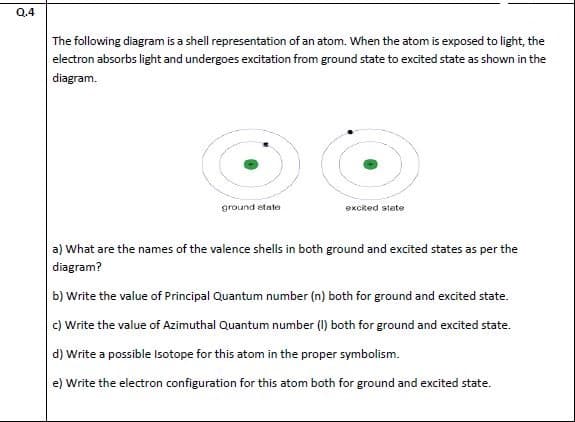
Transcribed Image Text:The following diagram is a shell representation of an atom. When the atom is exposed to light, the
electron absorbs light and undergoes excitation from ground state to excited state as shown in the
diagram.
ground etato
eKcked stete
Expert Solution
Step 1
a)
The outer most shell in which electron is present is known as valence shell.
For ground state = K is the valence shell
For excited state =L is the valence shell
b)
For ground state the value of Principal quantum number [n] = 1
For excited state the value of Principal quantum number [n] = 2
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co