The following information is given for mercury at latm: AHvap(357°C)= 59.3 kJ/mol boiling point 357 °C AHfus(-39 °C)= 2.33 melting point -39 °C kJ/mol specific heat solid= 0.141 J/goC specific heat liquid J/g°C 0.139 What is AH in kJ for the process of freezing a 49.0 g sample of liquid mercury at its normal melting point of -39 °C
The following information is given for mercury at latm: AHvap(357°C)= 59.3 kJ/mol boiling point 357 °C AHfus(-39 °C)= 2.33 melting point -39 °C kJ/mol specific heat solid= 0.141 J/goC specific heat liquid J/g°C 0.139 What is AH in kJ for the process of freezing a 49.0 g sample of liquid mercury at its normal melting point of -39 °C
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.6: Reaction Enthalpies For Chemical Reactions
Problem 4.7PSP: The reaction enthalpy for sublimation of 1 mol solid iodine at 25 C and 1 bar is 62.4 kJ....
Related questions
Question
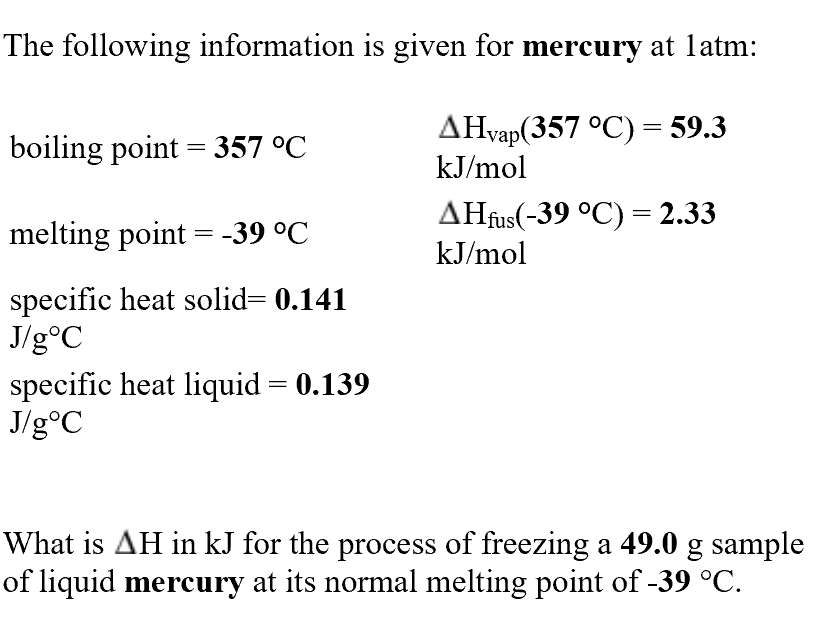
Transcribed Image Text:The following information is given for mercury at latm:
AHvap(357°C)= 59.3
kJ/mol
boiling point 357 °C
AHfus(-39 °C)= 2.33
melting point
-39 °C
kJ/mol
specific heat solid= 0.141
J/goC
specific heat liquid
J/g°C
0.139
What is AH in kJ for the process of freezing a 49.0 g sample
of liquid mercury at its normal melting point of -39 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning