The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, indluding physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. x10 2. Suppose 22.0 mL of dioxygen gas are produced by this reaction, at a temperature of 120.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Round your answer to 3 significant digits. Ig Check Explanation Terms of L O 2019 McGraw-Hill Education. All Rights Reserved. 99+ Type here to search hp delete prt sc fio fg
The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, indluding physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. x10 2. Suppose 22.0 mL of dioxygen gas are produced by this reaction, at a temperature of 120.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Round your answer to 3 significant digits. Ig Check Explanation Terms of L O 2019 McGraw-Hill Education. All Rights Reserved. 99+ Type here to search hp delete prt sc fio fg
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.141QP: A power plant is driven by the combustion of a complex fossil fuel having the formula C11H7S. Assume...
Related questions
Question
100%
question 2 please
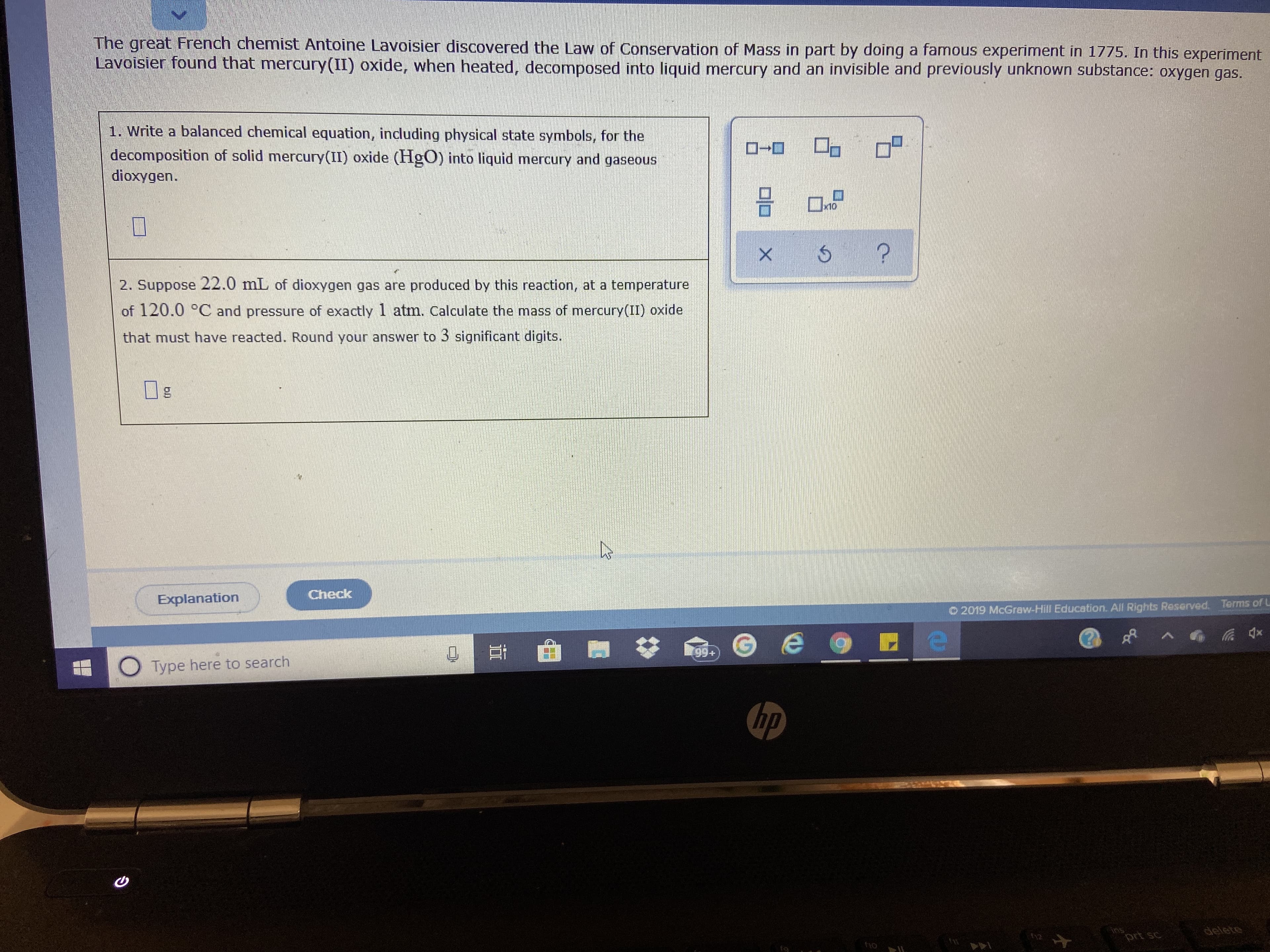
Transcribed Image Text:The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment
Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas.
1. Write a balanced chemical equation, indluding physical state symbols, for the
decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous
dioxygen.
x10
2. Suppose 22.0 mL of dioxygen gas are produced by this reaction, at a temperature
of 120.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide
that must have reacted. Round your answer to 3 significant digits.
Ig
Check
Explanation
Terms of L
O 2019 McGraw-Hill Education. All Rights Reserved.
99+
Type here to search
hp
delete
prt sc
fio
fg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning