The ground-state electron configuration of the H; molec- ular ion is (og15)'. (a) A molecule of H absorbs a photon, promoting the electron to the os molecular orbital. Predict what happens to the molecule. (b) Another molecule of H absorbs even more energy promoting the electron to the o,2, molecular orbital. Predict what happens to this molecule.
The ground-state electron configuration of the H; molec- ular ion is (og15)'. (a) A molecule of H absorbs a photon, promoting the electron to the os molecular orbital. Predict what happens to the molecule. (b) Another molecule of H absorbs even more energy promoting the electron to the o,2, molecular orbital. Predict what happens to this molecule.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 63P: The pyridine molecule (C5H5N) is obtained by replacing one CH group in benzene with a nitrogen atom....
Related questions
Question
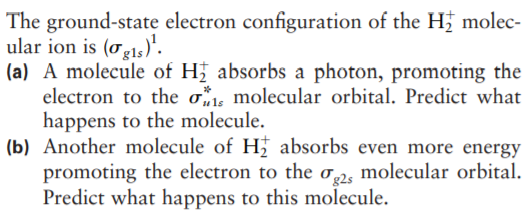
Transcribed Image Text:The ground-state electron configuration of the H; molec-
ular ion is (og15)'.
(a) A molecule of H absorbs a photon, promoting the
electron to the os molecular orbital. Predict what
happens to the molecule.
(b) Another molecule of H absorbs even more energy
promoting the electron to the o,2, molecular orbital.
Predict what happens to this molecule.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning