The ground-state wave function for the electron in a hydro- gen atom is 1 1,(7) = VTa where r is the radial coordinate of the electron and a, is the Bohr radius. (a) Show that the wave function as given is normalized. (b) Find the probability of locating the electron between r, = a,/2 and r, = 3a,/2.
The ground-state wave function for the electron in a hydro- gen atom is 1 1,(7) = VTa where r is the radial coordinate of the electron and a, is the Bohr radius. (a) Show that the wave function as given is normalized. (b) Find the probability of locating the electron between r, = a,/2 and r, = 3a,/2.
Related questions
Question
100%
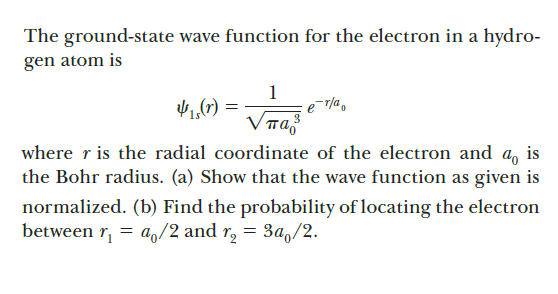
Transcribed Image Text:The ground-state wave function for the electron in a hydro-
gen atom is
1
1,(7) =
VTa
where r is the radial coordinate of the electron and a, is
the Bohr radius. (a) Show that the wave function as given is
normalized. (b) Find the probability of locating the electron
between r,
= a,/2 and r, = 3a,/2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
