The H/CO na in matures of carbon monoxide and hydrogen (rated synthesis gas) is increased by the water gas shit reaction CO(g) + H₂O(g) CO(g) + Hy(9), which has an equibrum constant K. - 4.24 at 800 I Part A Calculate the equilibrum concentrations of CO₂, H₂, CO, and H₂O at 800 K if only CO and H₂O are present initially at concentrations of 0.118 M Express your answers using three decimal places separated by commas VAZO (col. ₂. (CO), H₂0- Submit Previs Anewers en Provide feedbac 946 ? M Next >
The H/CO na in matures of carbon monoxide and hydrogen (rated synthesis gas) is increased by the water gas shit reaction CO(g) + H₂O(g) CO(g) + Hy(9), which has an equibrum constant K. - 4.24 at 800 I Part A Calculate the equilibrum concentrations of CO₂, H₂, CO, and H₂O at 800 K if only CO and H₂O are present initially at concentrations of 0.118 M Express your answers using three decimal places separated by commas VAZO (col. ₂. (CO), H₂0- Submit Previs Anewers en Provide feedbac 946 ? M Next >
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.95QE: Nitrogen, hydrogen, and ammonia are in equilibrium in a 1000-L reactor at 550 K. The concentration...
Related questions
Question
Give handwritten answer
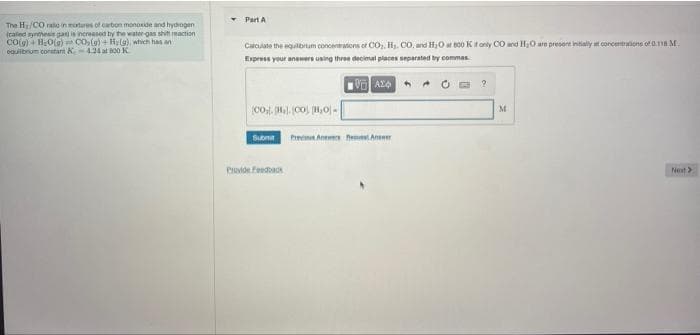
Transcribed Image Text:The H/CO rato in motures of carbon monoxide and hydrogen
(raled synthesis gas) is increased by the water gas shit reaction
CO(g) + H₂O(g) CO₂(g) + Hy(g), which has an
equilibrium constan K 4.24 at 800 K
Part A
Calculate the equilibrum concentrations of CO₂, H₂, CO. and H₂O at 800 K if only CO and H₂O are present initially at concentrations of 0.118 M.
Express your answers using three decimal places separated by commas
VAX
©Oị. {H%. CO\ [B,O| «
Submit Prev Anews An
Provide feedback
?
M
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning