The illustration on the left represents a mixture of I2 (purple) molecules and F2 (green) molecules If these were to react to form IF3 molecules, what is the maximum number of IF3 molecules that could form? Hint: Atoms must be conserved in an ordinary chemical reaction IF3 molecules The balanced chemical equation for the reaction between sodium and water is: 2Na(s)2H2O(I) -2N2OH(aq) H2(g) We can interpret this to mean and |moles of water 2 moles of sodium React to produce moles of sodium hydroxide and moles of hydrogen
The illustration on the left represents a mixture of I2 (purple) molecules and F2 (green) molecules If these were to react to form IF3 molecules, what is the maximum number of IF3 molecules that could form? Hint: Atoms must be conserved in an ordinary chemical reaction IF3 molecules The balanced chemical equation for the reaction between sodium and water is: 2Na(s)2H2O(I) -2N2OH(aq) H2(g) We can interpret this to mean and |moles of water 2 moles of sodium React to produce moles of sodium hydroxide and moles of hydrogen
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 21ALQ: Atoms of three different elements are represented by O, , and . Which compound is left over when...
Related questions
Question
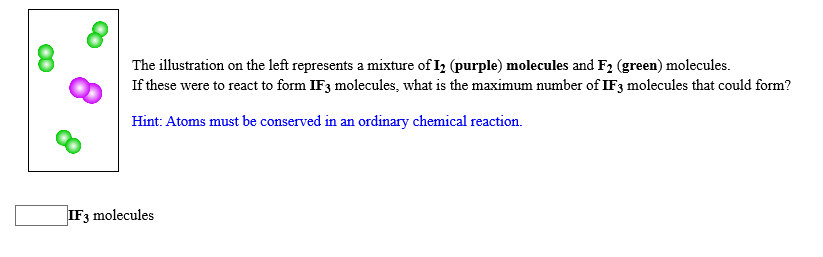
Transcribed Image Text:The illustration on the left represents a mixture of I2 (purple) molecules and F2 (green) molecules
If these were to react to form IF3 molecules, what is the maximum number of IF3 molecules that could form?
Hint: Atoms must be conserved in an ordinary chemical reaction
IF3 molecules
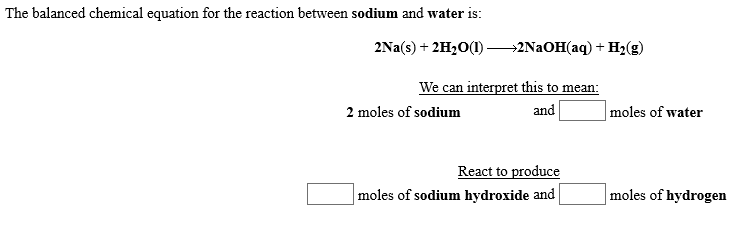
Transcribed Image Text:The balanced chemical equation for the reaction between sodium and water is:
2Na(s)2H2O(I)
-2N2OH(aq) H2(g)
We can interpret this to mean
and
|moles of water
2 moles of sodium
React to produce
moles of sodium hydroxide and
moles of hydrogen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning