The isoelectric point, pl, of the protein nitrate reductase is 4.2 , while that of superoxide dismutase is 4.95 . What is the net charge of nitrate reductase at pH 5.1 ?| What is the net charge of superoxide dismutase at pH 6.5 ? The isoelectric point of phenylalanine is 5.48 ; leucine , 5.98 . During paper electrophoresis at pH 4.5 , toward which electrode does phenylalanine migrate? During paper electrophoresis at pH 5.5 , toward which electrode does leucine migrate?|
The isoelectric point, pl, of the protein nitrate reductase is 4.2 , while that of superoxide dismutase is 4.95 . What is the net charge of nitrate reductase at pH 5.1 ?| What is the net charge of superoxide dismutase at pH 6.5 ? The isoelectric point of phenylalanine is 5.48 ; leucine , 5.98 . During paper electrophoresis at pH 4.5 , toward which electrode does phenylalanine migrate? During paper electrophoresis at pH 5.5 , toward which electrode does leucine migrate?|
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter5: Measurements And Calculations
Section5.2: Uncertainty In Measurement And Significant Figures
Problem 6RQ
Related questions
Question
Please answer with positive or negative, thanks!
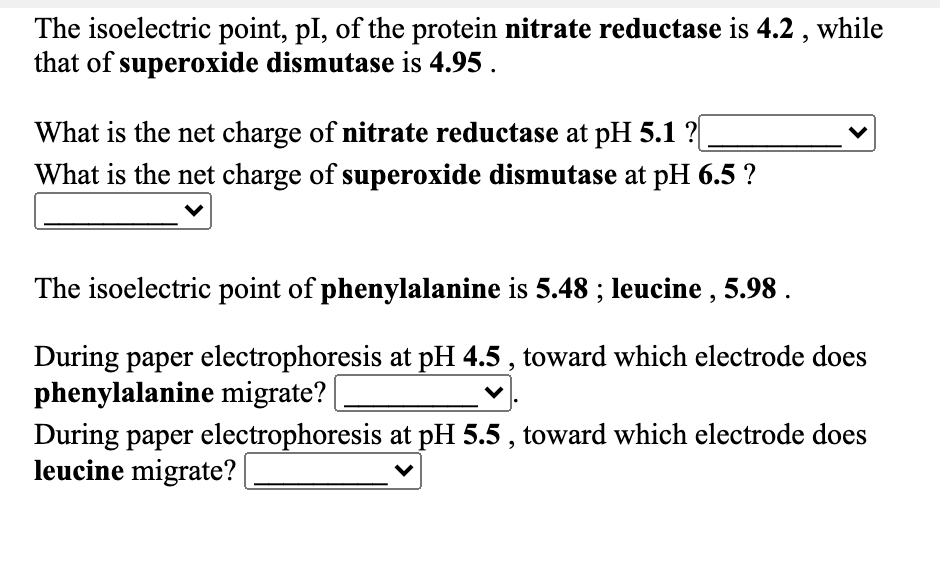
Transcribed Image Text:The isoelectric point, pl, of the protein nitrate reductase is 4.2 , while
that of superoxide dismutase is 4.95 .
What is the net charge of nitrate reductase at pH 5.1 ?|
What is the net charge of superoxide dismutase at pH 6.5 ?
The isoelectric point of phenylalanine is 5.48 ; leucine , 5.98 .
During paper electrophoresis at pH 4.5 , toward which electrode does
phenylalanine migrate?
During paper electrophoresis at pH 5.5 , toward which electrode does
leucine migrate?|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning