The mass unit associated with density is usually grams. If the volume (in mL or cm) is multiplied by the density (g/mL or g/cm³) the volume units will cancel out, leaving only the mass units. Keep in mind that the volume and density must use the same volume unit for the cancellation. If a large marshmallow has a volume of 2.00 in³ and density of 0.242 g/cm³, how much would it weigh in grams? 1 in3 = 16.39 cm³. Express your answer in grams to three significant figures. • View Available Hint(s) ν ΑΣφ ? mass of marshmallow = g
The mass unit associated with density is usually grams. If the volume (in mL or cm) is multiplied by the density (g/mL or g/cm³) the volume units will cancel out, leaving only the mass units. Keep in mind that the volume and density must use the same volume unit for the cancellation. If a large marshmallow has a volume of 2.00 in³ and density of 0.242 g/cm³, how much would it weigh in grams? 1 in3 = 16.39 cm³. Express your answer in grams to three significant figures. • View Available Hint(s) ν ΑΣφ ? mass of marshmallow = g
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
ChapterA: Scientific Notation And Experimental Error
Section: Chapter Questions
Problem 10P
Related questions
Question
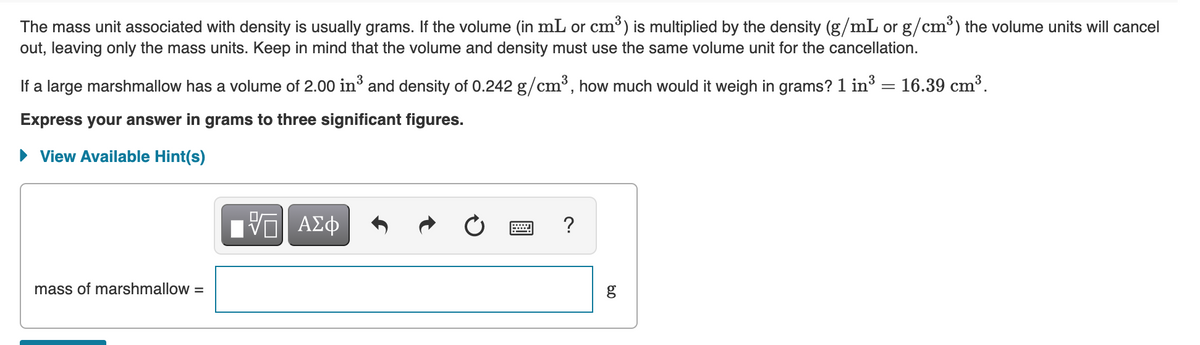
Transcribed Image Text:The mass unit associated with density is usually grams. If the volume (in mL or cm³) is multiplied by the density (g/mL or g/cm³) the volume units will cancel
out, leaving only the mass units. Keep in mind that the volume and density must use the same volume unit for the cancellation.
If a large marshmallow has a volume of 2.00 in³ and density of 0.242 g/cm³, how much would it weigh in grams? 1 in3
16.39 cm³.
Express your answer in grams to three significant figures.
• View Available Hint(s)
ΑΣφ
?
DA
mass of marshmallow =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning