The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). -6 mol/L. Note: "major" chemical species are those present in concentrations greater than 10 major species present when dissolved in water compound formula FeCl, iron(III) chloride AGNO, silver nitrate C12 H22011 sucrose I Don't Know Submit | Privacy Terms of Use © 2020 McGraw-Hill Education. All Rights Reserved. JAN O Ctv 8. MacBook Pr esc & @ 4 3 K H. в option command ption command 00
The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). -6 mol/L. Note: "major" chemical species are those present in concentrations greater than 10 major species present when dissolved in water compound formula FeCl, iron(III) chloride AGNO, silver nitrate C12 H22011 sucrose I Don't Know Submit | Privacy Terms of Use © 2020 McGraw-Hill Education. All Rights Reserved. JAN O Ctv 8. MacBook Pr esc & @ 4 3 K H. в option command ption command 00
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.4QE
Related questions
Question
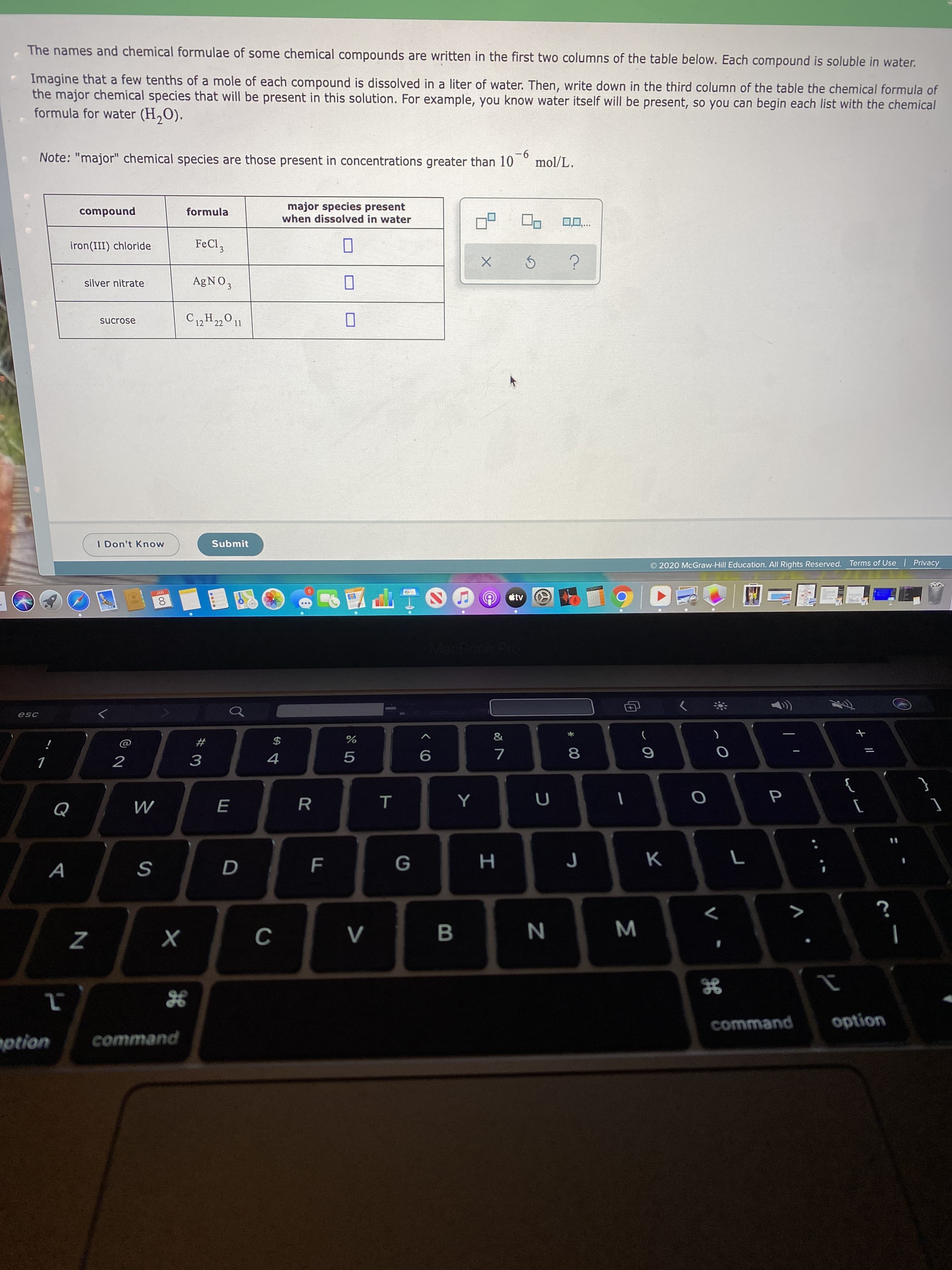
Transcribed Image Text:The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of
the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical
formula for water (H,O).
-6
mol/L.
Note: "major" chemical species are those present in concentrations greater than 10
major species present
when dissolved in water
compound
formula
FeCl,
iron(III) chloride
AGNO,
silver nitrate
C12 H22011
sucrose
I Don't Know
Submit
| Privacy
Terms of Use
© 2020 McGraw-Hill Education. All Rights Reserved.
JAN
O Ctv
8.
MacBook Pr
esc
&
@
4
3
K
H.
в
option
command
ption
command
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning