The number of electrons excited to the conduction band per cubic centimeter in a semiconductor can be estimated from the following equation: ne = (4.8 × 10'5 cm¯³ K¯¥2) T/2 e¯E{2RT) K¯32) T³² e¯E«M2RT) п where T is the temperature in kelvins and Eg the band gap in joules per mole. The band gap of diamond at 300 K is 8.7 × 10-19 J. How many electrons are thermally excited to the conduction band at this temperature in a 1.00-cm diamond crystal?
The number of electrons excited to the conduction band per cubic centimeter in a semiconductor can be estimated from the following equation: ne = (4.8 × 10'5 cm¯³ K¯¥2) T/2 e¯E{2RT) K¯32) T³² e¯E«M2RT) п where T is the temperature in kelvins and Eg the band gap in joules per mole. The band gap of diamond at 300 K is 8.7 × 10-19 J. How many electrons are thermally excited to the conduction band at this temperature in a 1.00-cm diamond crystal?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.30PAE
Related questions
Question
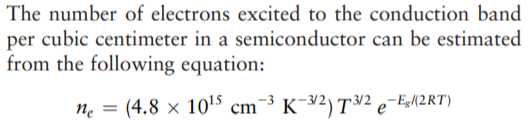
Transcribed Image Text:The number of electrons excited to the conduction band
per cubic centimeter in a semiconductor can be estimated
from the following equation:
ne = (4.8 × 10'5 cm¯³ K¯¥2) T/2 e¯E{2RT)
K¯32) T³² e¯E«M2RT)
п

Transcribed Image Text:where T is the temperature in kelvins and Eg the band gap
in joules per mole. The band gap of diamond at 300 K is
8.7 × 10-19 J. How many electrons are thermally excited
to the conduction band at this temperature in a 1.00-cm
diamond crystal?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
