The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water. What is the maximum mass of H,0 that can be produced by combining 81.4 g of each reactant? 4 NH, (g) + 50,(g) → 4 NO(g) + 6 H,O(g) g H,O mass:
The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water. What is the maximum mass of H,0 that can be produced by combining 81.4 g of each reactant? 4 NH, (g) + 50,(g) → 4 NO(g) + 6 H,O(g) g H,O mass:
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 121E: Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane:...
Related questions
Question
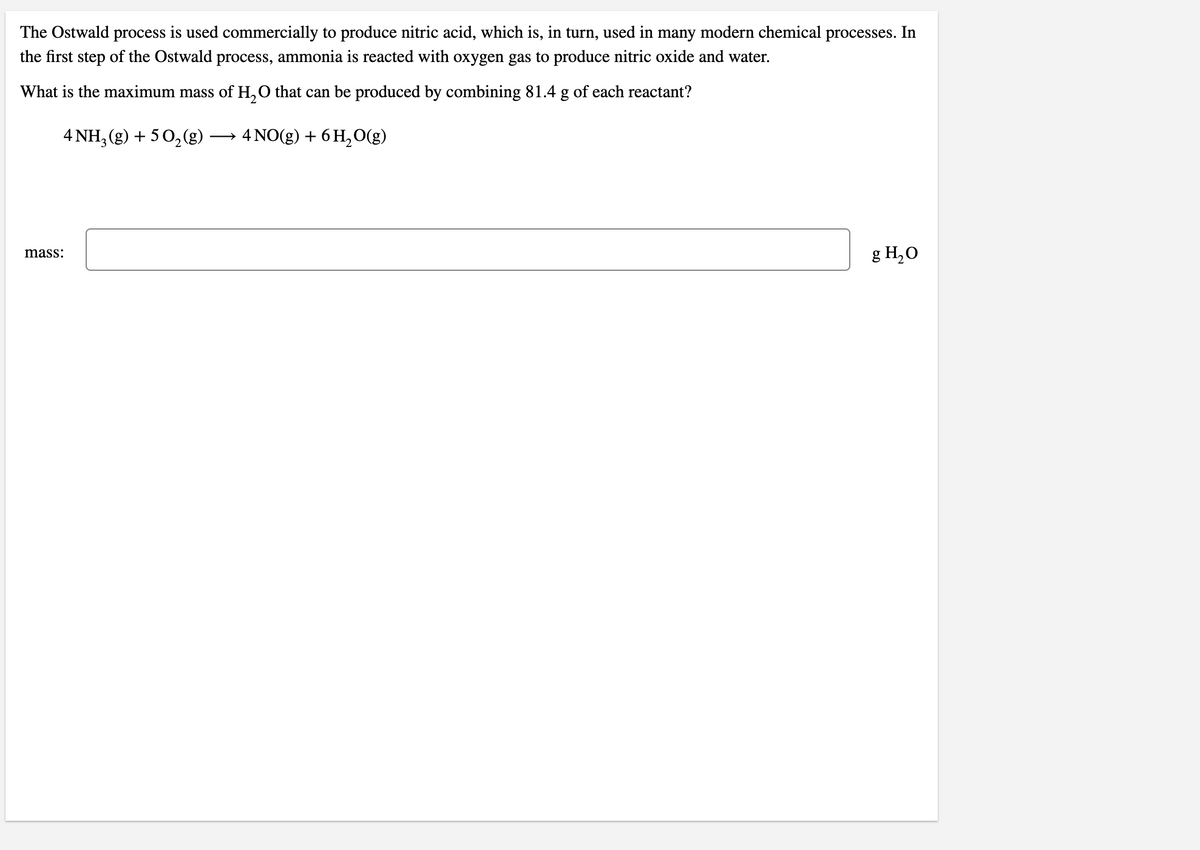
Transcribed Image Text:The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In
the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water.
What is the maximum mass of H,O that can be produced by combining 81.4 g of each reactant?
4 NH, (g) + 50,(g)
4 NO(g) + 6 H, O(g)
>
g H,O
mass:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning