The pka of phenol (C6H5OH) is 10.0. When a nitro group (NO2) is attached to the ring, the pK, decreases, as shown for the ortho, meta, and para isomers. OH OH ОН OH NO2 (a) Explain why the pK, values of all three isomers are lower than the pka of phenol itself. (b) Explain why the meta isomer has the highest pka of `NO2 Phenol NO2 the three isomers. pKa = 10.0 7.23 8.35 7.14
The pka of phenol (C6H5OH) is 10.0. When a nitro group (NO2) is attached to the ring, the pK, decreases, as shown for the ortho, meta, and para isomers. OH OH ОН OH NO2 (a) Explain why the pK, values of all three isomers are lower than the pka of phenol itself. (b) Explain why the meta isomer has the highest pka of `NO2 Phenol NO2 the three isomers. pKa = 10.0 7.23 8.35 7.14
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 25VC: The following molecular model of a dimethyl-substituted biphenyl represents the lowest-energy...
Related questions
Question
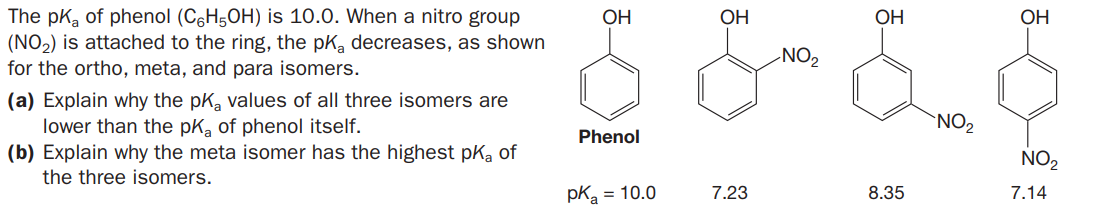
Transcribed Image Text:The pka of phenol (C6H5OH) is 10.0. When a nitro group
(NO2) is attached to the ring, the pK, decreases, as shown
for the ortho, meta, and para isomers.
OH
OH
ОН
OH
NO2
(a) Explain why the pK, values of all three isomers are
lower than the pka of phenol itself.
(b) Explain why the meta isomer has the highest pka of
`NO2
Phenol
NO2
the three isomers.
pKa = 10.0
7.23
8.35
7.14
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

