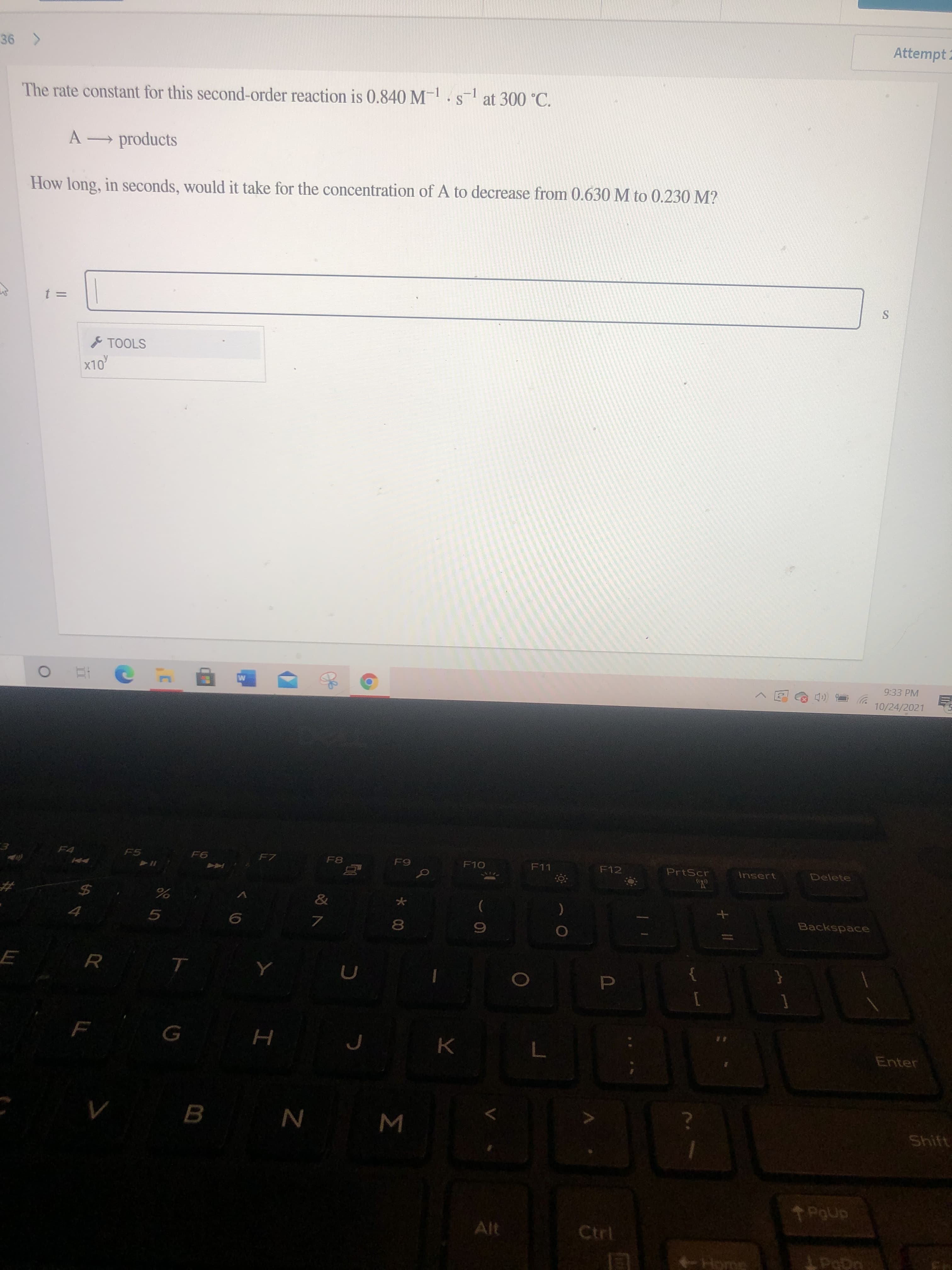
The rate constant for this second-order reaction is 0.840 M-.s at 300 °C. A products How long, in seconds, would it take for the concentration of A to decrease from 0.630 M to 0.230 M?
The rate constant for this second-order reaction is 0.840 M-.s at 300 °C. A products How long, in seconds, would it take for the concentration of A to decrease from 0.630 M to 0.230 M?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 22QAP: The hypothetical reaction X(g)+12Y(g)productsis first-order in X and second-order in Y. The rate of...
Related questions
Question
Transcribed Image Text:P
* 00
Attempt 2
The rate constant for this second-order reaction is 0.840 M-.s at 300 °C.
-1
S
A products
How long, in seconds, would it take for the concentration of A to decrease from 0.630 M to 0.230 M?
& TOOLS
x10
OLX
9:33 PM
10/24/2021
F7
F10
F12
PrtScr
Insert
Delete
&
24
Backspace
5.
R
Enter
K.
B.
Shift
W N
Alt
Ctrl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
