The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG° stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to it. Otherwise, chedk the "no false statements" box under the table statement false? statement false? X 5? In K< 0 In K 0 K> 1 K=1 AG° <0 AG 0 ΔΗ ΤΔS. ΔΗ ΤΔS* no false statements: no false statements: Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Pri Lenovo Insert Home End I Esc F11 F12 F9 F10 F8 F7 F6 F5 F4 F2 F3 FnLock F1 & # 7 4 5 3 1 2 II CO LO
The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG° stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to it. Otherwise, chedk the "no false statements" box under the table statement false? statement false? X 5? In K< 0 In K 0 K> 1 K=1 AG° <0 AG 0 ΔΗ ΤΔS. ΔΗ ΤΔS* no false statements: no false statements: Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Pri Lenovo Insert Home End I Esc F11 F12 F9 F10 F8 F7 F6 F5 F4 F2 F3 FnLock F1 & # 7 4 5 3 1 2 II CO LO
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.64P: 7-64 As we shall see in Chapter 20, there are two forms of glucose, designated alpha and betawhich...
Related questions
Question
And each table there may be one statement that is false because it contradicts the other three statements if you find a false statement check the box next to it otherwise check the no-fault state in box under the table. Please check your answers
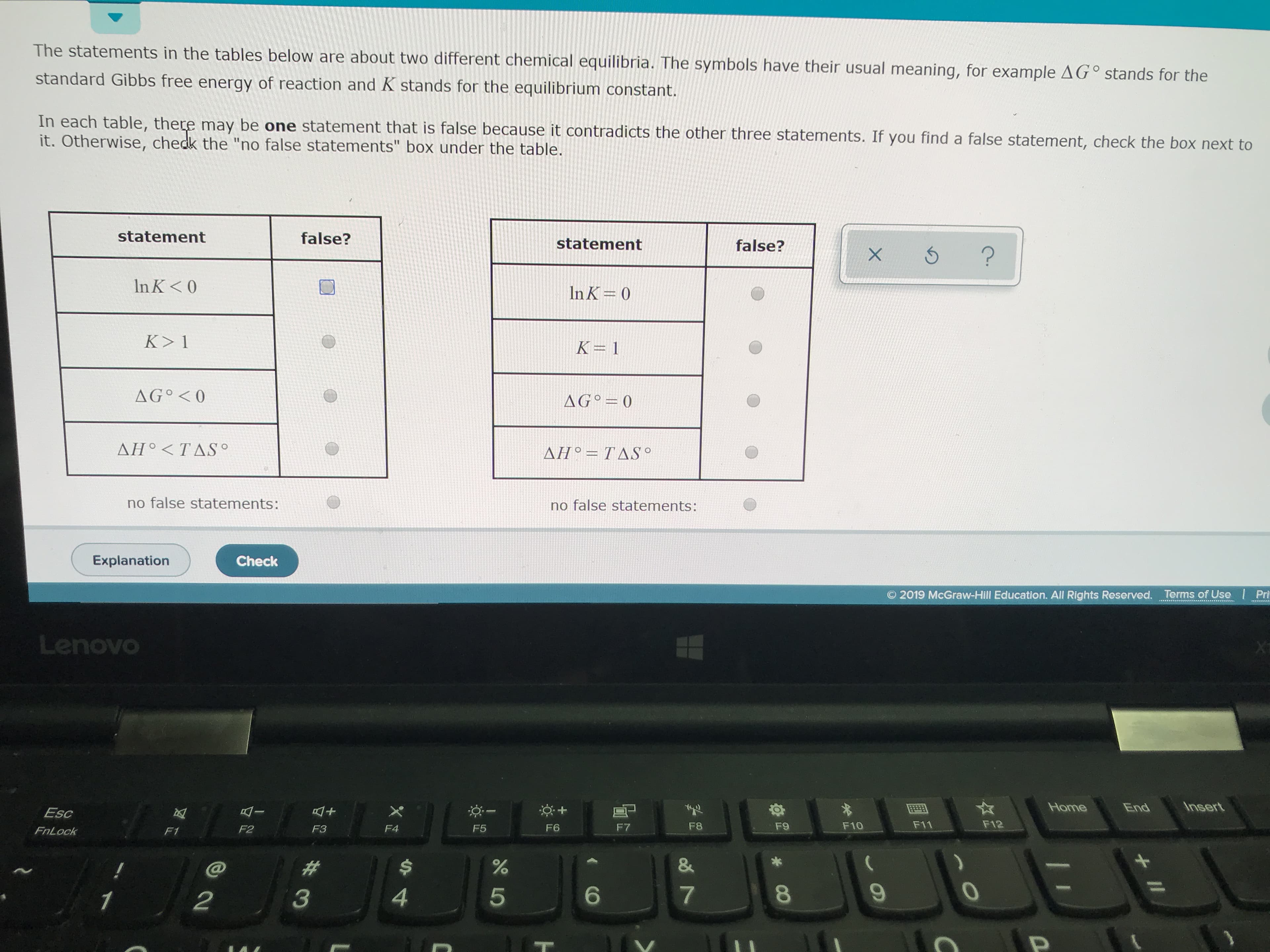
Transcribed Image Text:The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG° stands for the
standard Gibbs free energy of reaction and K stands for the equilibrium constant
In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to
it. Otherwise, chedk the "no false statements" box under the table
statement
false?
statement
false?
X 5?
In K< 0
In K 0
K> 1
K=1
AG° <0
AG 0
ΔΗ ΤΔS.
ΔΗ ΤΔS*
no false statements:
no false statements:
Explanation
Check
2019 McGraw-Hill Education. All Rights Reserved. Terms of Use
Pri
Lenovo
Insert
Home
End
I
Esc
F11
F12
F9
F10
F8
F7
F6
F5
F4
F2
F3
FnLock
F1
&
#
7
4
5
3
1
2
II
CO
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning