The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). The reaction between the acid and the zinc is as follows: Part A 2H (aq)Zn(s) -> H2(g) Zn2(aq) What mass of hydrogen gas was collected? When the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °C was 0.957 L at a total pressure of 758 mm Hg . VASD η ΑΣφ Vapor Pressure of Water versus Temperature Temperature Pressure Temperature Pressure (mmHg) (° C) m = g (C) (mmHg) 4.58 118.2 0 55 Request Answer Submit 6.54 60 149.6 9.21 65 187.5 10 233.7 15 12.79 70 Return to Assignment Provide Feedback 20 17.55 289.1 75 25 23.78 355.1 80 85 433.6 30 31.86 35 42.23 525.8 90 40 55.40 633.9 95 45 760.0 71.97 100 92.6 50
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). The reaction between the acid and the zinc is as follows: Part A 2H (aq)Zn(s) -> H2(g) Zn2(aq) What mass of hydrogen gas was collected? When the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °C was 0.957 L at a total pressure of 758 mm Hg . VASD η ΑΣφ Vapor Pressure of Water versus Temperature Temperature Pressure Temperature Pressure (mmHg) (° C) m = g (C) (mmHg) 4.58 118.2 0 55 Request Answer Submit 6.54 60 149.6 9.21 65 187.5 10 233.7 15 12.79 70 Return to Assignment Provide Feedback 20 17.55 289.1 75 25 23.78 355.1 80 85 433.6 30 31.86 35 42.23 525.8 90 40 55.40 633.9 95 45 760.0 71.97 100 92.6 50
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.122QE: A Liquid HF undergoes an autoionization reaction: 2HFH2F++F (a) Is KF an acid or a base in this...
Related questions
Question
100%
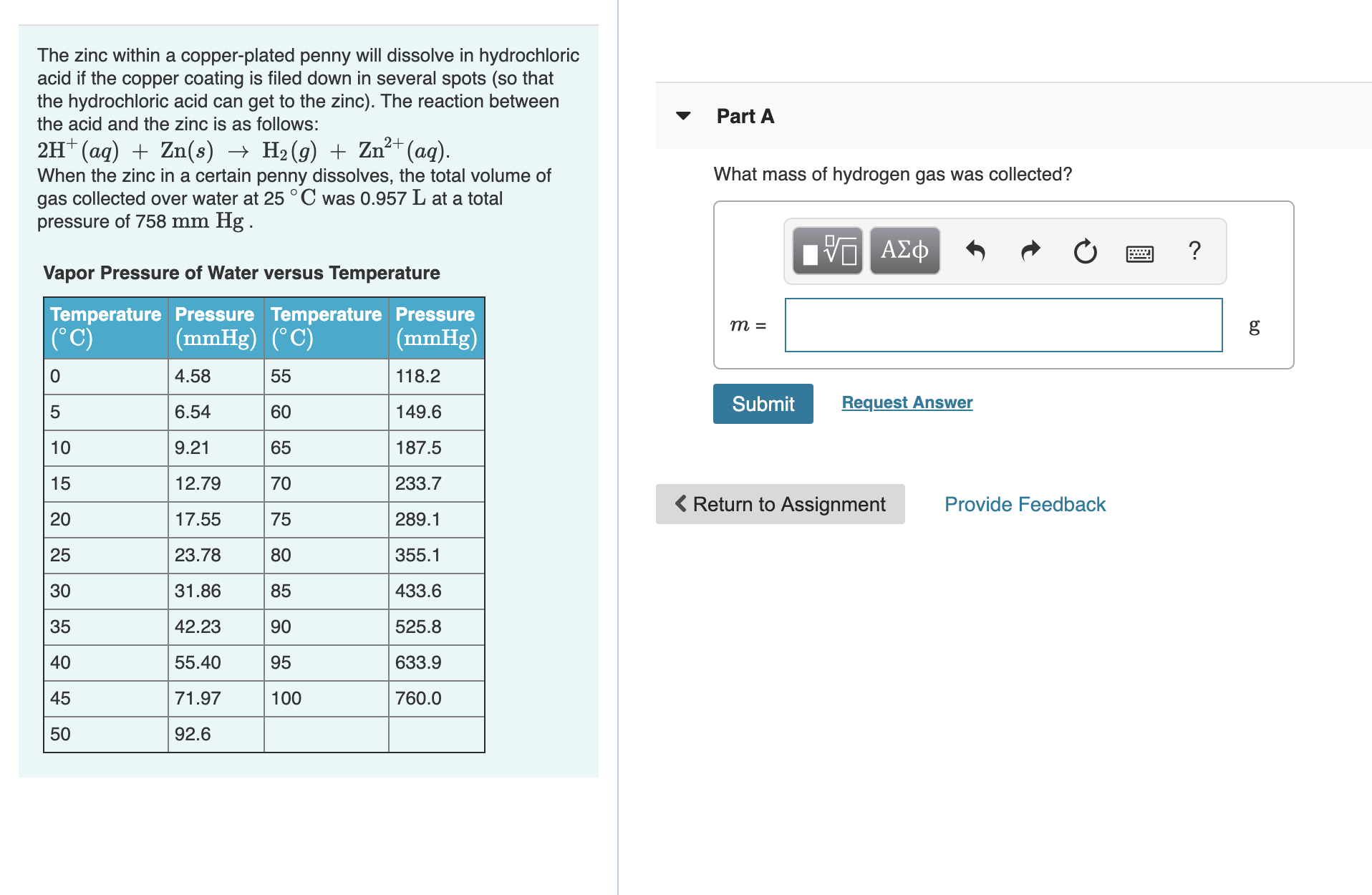
Transcribed Image Text:The zinc within a copper-plated penny will dissolve in hydrochloric
acid if the copper coating is filed down in several spots (so that
the hydrochloric acid can get to the zinc). The reaction between
the acid and the zinc is as follows:
Part A
2H (aq)Zn(s) -> H2(g) Zn2(aq)
What mass of hydrogen gas was collected?
When the zinc in a certain penny dissolves, the total volume of
gas collected over water at 25 °C was 0.957 L at a total
pressure of 758 mm Hg .
VASD
η ΑΣφ
Vapor Pressure of Water versus Temperature
Temperature Pressure Temperature Pressure
(mmHg) (° C)
m =
g
(C)
(mmHg)
4.58
118.2
0
55
Request Answer
Submit
6.54
60
149.6
9.21
65
187.5
10
233.7
15
12.79
70
Return to Assignment
Provide Feedback
20
17.55
289.1
75
25
23.78
355.1
80
85
433.6
30
31.86
35
42.23
525.8
90
40
55.40
633.9
95
45
760.0
71.97
100
92.6
50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning