There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC2(s) + 2 H20(g)C2H2(g) Ca(OH)2(s) AH=-414. kJ In the second step, acetylene, carbon dioxide and water react to form acrylic acid : 6 C2H2(9) + 3 CO2(g) + 4H20(g)5 CH2CHCO2H(g) AH=132. kJ Calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. Round your answer to the nearest kJ. kJ ? X Submit Assignment Continue Privacy Terms of Use 2019 McGraw-Hill Education. All Rights Reserved. DD DII O00 O00 F10 F9 F8 F7 esc F6 F 5 F 4 F3 F2 F1 00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: CaC2(s) + 2 H20(g)C2H2(g) Ca(OH)2(s) AH=-414. kJ In the second step, acetylene, carbon dioxide and water react to form acrylic acid : 6 C2H2(9) + 3 CO2(g) + 4H20(g)5 CH2CHCO2H(g) AH=132. kJ Calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. Round your answer to the nearest kJ. kJ ? X Submit Assignment Continue Privacy Terms of Use 2019 McGraw-Hill Education. All Rights Reserved. DD DII O00 O00 F10 F9 F8 F7 esc F6 F 5 F 4 F3 F2 F1 00
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 117E: Bacterial digestion is an economical method of sewage treatment. The reaction is an intermediate...
Related questions
Question
100%
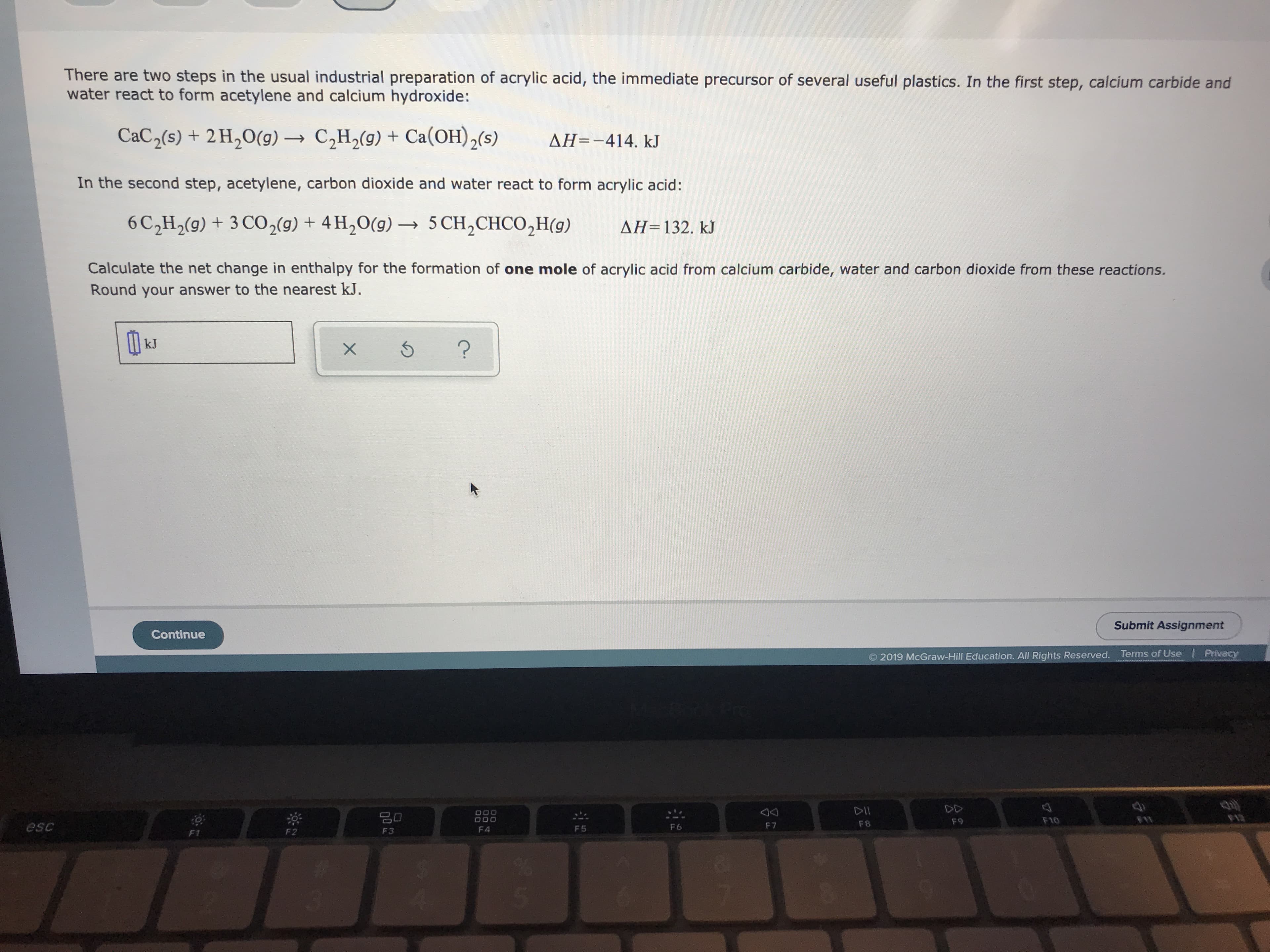
Transcribed Image Text:There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and
water react to form acetylene and calcium hydroxide:
CaC2(s) + 2 H20(g)C2H2(g) Ca(OH)2(s)
AH=-414. kJ
In the second step, acetylene, carbon dioxide and water react to form acrylic acid :
6 C2H2(9) + 3 CO2(g) + 4H20(g)5 CH2CHCO2H(g)
AH=132. kJ
Calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions.
Round your answer to the nearest kJ.
kJ
?
X
Submit Assignment
Continue
Privacy
Terms of Use
2019 McGraw-Hill Education. All Rights Reserved.
DD
DII
O00
O00
F10
F9
F8
F7
esc
F6
F 5
F 4
F3
F2
F1
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning