Three major contributing resonance structures are possible for the following cation. One is given below. Draw the remaining structures (in any order), including nonbonding electrons and formal charges. Omit curved arrows. н н CH3З Which contributes most to the hybrid? The structure with the positive charge on sulfur. All contribute equally. O The structures with the positive charge on carbon.
Three major contributing resonance structures are possible for the following cation. One is given below. Draw the remaining structures (in any order), including nonbonding electrons and formal charges. Omit curved arrows. н н CH3З Which contributes most to the hybrid? The structure with the positive charge on sulfur. All contribute equally. O The structures with the positive charge on carbon.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter9: Chemical Bonds
Section: Chapter Questions
Problem 9.65QE: Write all possible resonance structures for the following species. Assign a formal charge to each...
Related questions
Question
100%
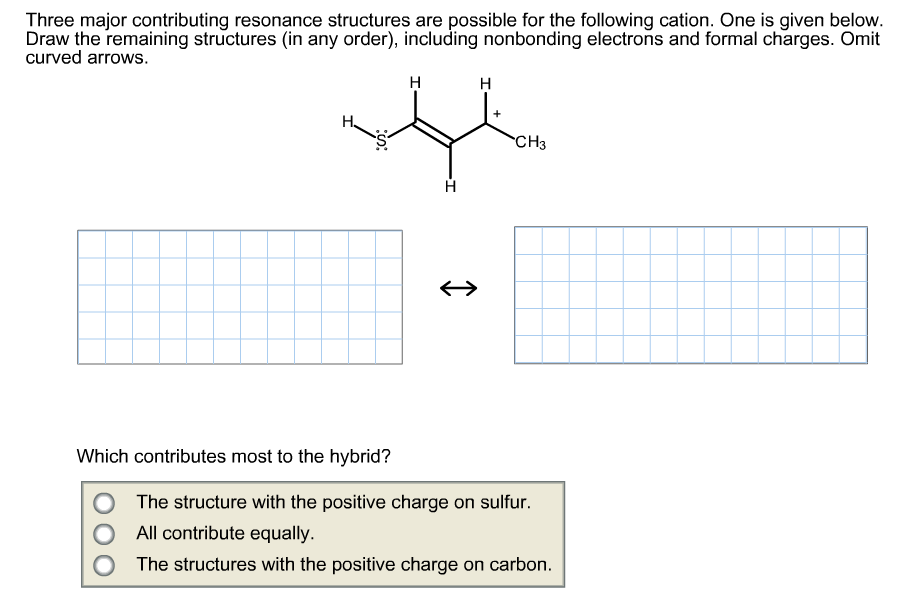
Transcribed Image Text:Three major contributing resonance structures are possible for the following cation. One is given below.
Draw the remaining structures (in any order), including nonbonding electrons and formal charges. Omit
curved arrows.
н
н
CH3З
Which contributes most to the hybrid?
The structure with the positive charge on sulfur.
All contribute equally.
O The structures with the positive charge on carbon.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning