To be metabolized, glucose must be converted to glucose 6-phosphate. However, the phosphorylation of glucose to form glucose 6-phosphate is endergonic with a positive AG. How do cells circumvent this problem? The reaction is catalyzed by an enzyme, which allows the reaction to proceed by lowering its activation energy. An enzyme raises the activation energy barrier to favor glucose 6-phosphate formation. The reaction is catalyzed by an enzyme, which allows the reaction to proceed by changing its AG. An enzyme changes the equilibrium constant for the reaction to favor glucose 6-phosphate formation. The reaction is coupled to the hydrolysis of ATP, making the entire process exergonic.
To be metabolized, glucose must be converted to glucose 6-phosphate. However, the phosphorylation of glucose to form glucose 6-phosphate is endergonic with a positive AG. How do cells circumvent this problem? The reaction is catalyzed by an enzyme, which allows the reaction to proceed by lowering its activation energy. An enzyme raises the activation energy barrier to favor glucose 6-phosphate formation. The reaction is catalyzed by an enzyme, which allows the reaction to proceed by changing its AG. An enzyme changes the equilibrium constant for the reaction to favor glucose 6-phosphate formation. The reaction is coupled to the hydrolysis of ATP, making the entire process exergonic.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter27: Metabolic Integration And Organ Specialization
Section: Chapter Questions
Problem 20P: Figure 27.3 illustrates the response of R (ATP-regenerating) and U (ATP-utilizing) enzymes to energy...
Related questions
Question
please see attached
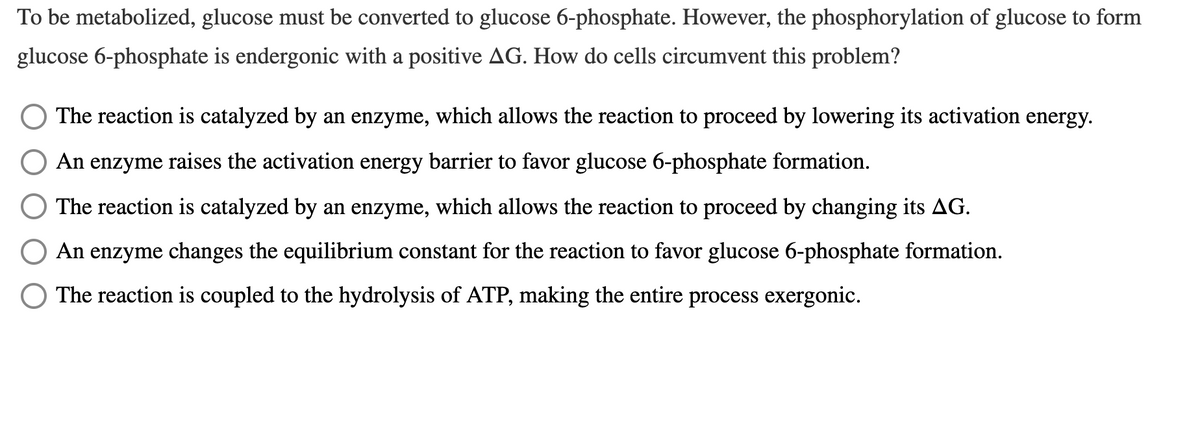
Transcribed Image Text:To be metabolized, glucose must be converted to glucose 6-phosphate. However, the phosphorylation of glucose to form
glucose 6-phosphate is endergonic with a positive AG. How do cells circumvent this problem?
The reaction is catalyzed by an enzyme, which allows the reaction to proceed by lowering its activation energy.
An enzyme raises the activation energy barrier to favor glucose 6-phosphate formation.
The reaction is catalyzed by an enzyme, which allows the reaction to proceed by changing its AG.
An enzyme changes the equilibrium constant for the reaction to favor glucose 6-phosphate formation.
The reaction is coupled to the hydrolysis of ATP, making the entire process exergonic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning