to lower-en ces photons of many different wavelengths (the d en spectrum) in the visible and other regions of th series lines occur in the ultraviolet region, the Bal en and Brackett are in the infrared. shown below can be used to calculate which tra photons we actually observe. 1 RH 2. ni nstant (1.097 × 107 m1) for hydrogen, n¡ is the lo itial or higher-energy level. nd Diffraction Film Power Supply and Hydrogen Tube ight bulb ht bul Red: RH 1.097x10m 606x10 M. ni° 0.1304...: -0.0995 =
to lower-en ces photons of many different wavelengths (the d en spectrum) in the visible and other regions of th series lines occur in the ultraviolet region, the Bal en and Brackett are in the infrared. shown below can be used to calculate which tra photons we actually observe. 1 RH 2. ni nstant (1.097 × 107 m1) for hydrogen, n¡ is the lo itial or higher-energy level. nd Diffraction Film Power Supply and Hydrogen Tube ight bulb ht bul Red: RH 1.097x10m 606x10 M. ni° 0.1304...: -0.0995 =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 33P
Related questions
Question
The measured wavelength is 606 nm.
What is the energy level transition (which shell is the electron coming from to get to n=2)?
See images
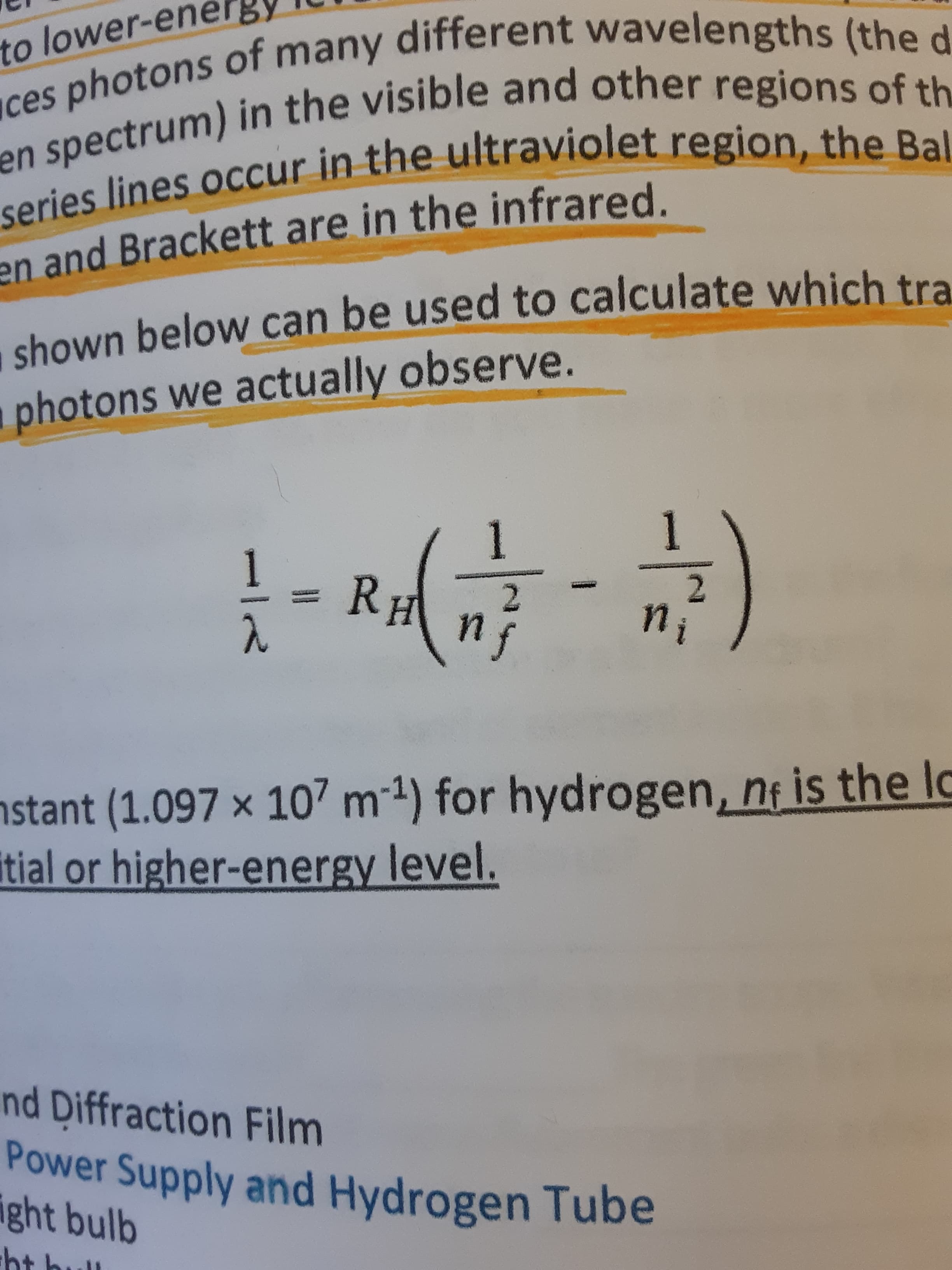
Transcribed Image Text:to lower-en
ces photons of many different wavelengths (the d
en spectrum) in the visible and other regions of th
series lines occur in the ultraviolet region, the Bal
en and Brackett are in the infrared.
shown below can be used to calculate which tra
photons we actually observe.
1
RH
2.
ni
nstant (1.097 × 107 m1) for hydrogen, n¡ is the lo
itial or higher-energy level.
nd Diffraction Film
Power Supply and Hydrogen Tube
ight bulb
ht bul
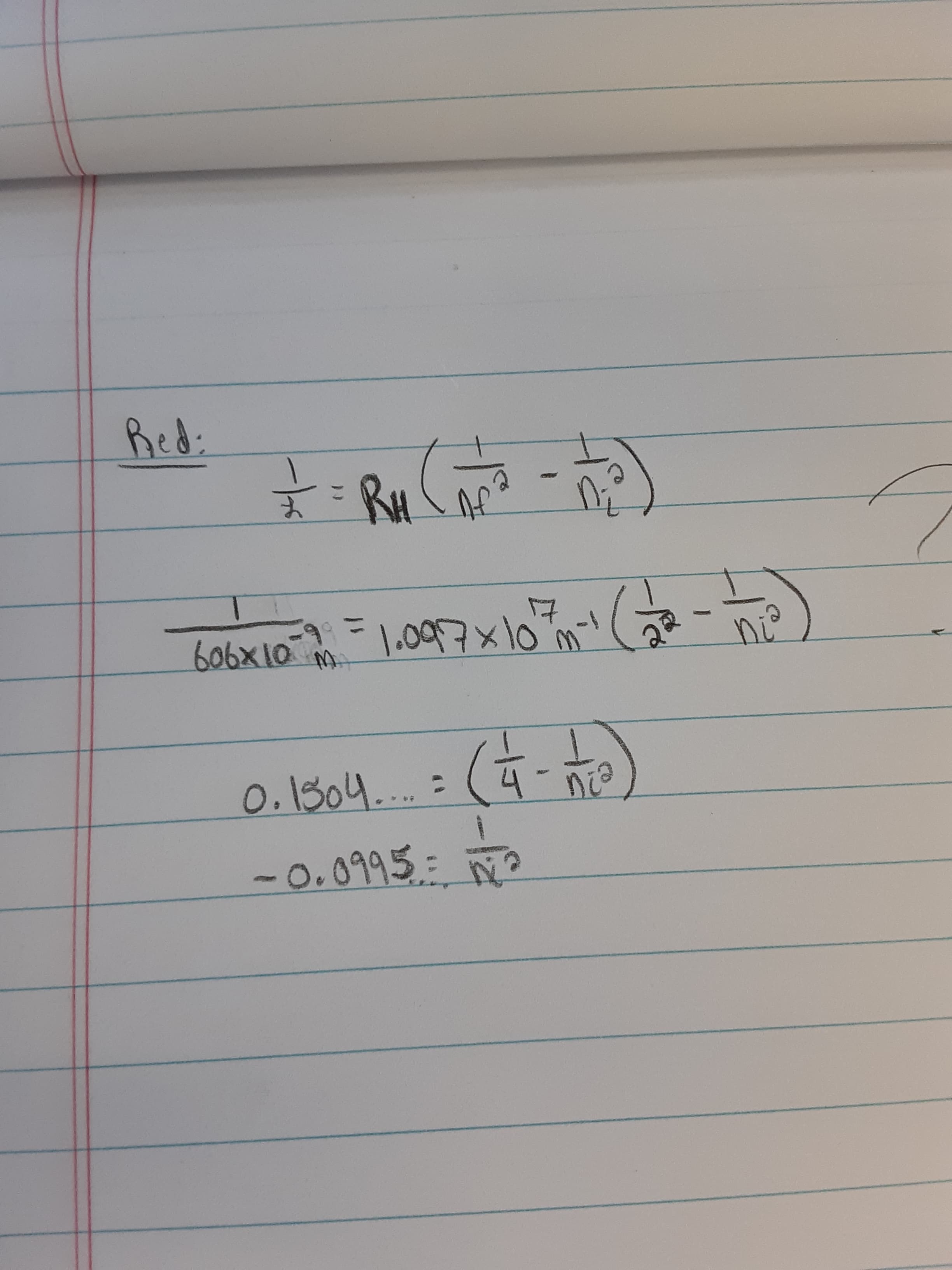
Transcribed Image Text:Red:
RH
1.097x10m
606x10 M.
ni°
0.1304...:
-0.0995
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
