Treatment of propadiene (an allene) with hydrogen bromide produces 2-bromopropene as the major product. This suggests that the more stable carbocation intermediate is produced by the addition of a proton to Br HBr. H2C=C=CH, H3C CH2 a terminal carbon rather than to the central carbon. Propadiene 2-Bromopropene (a) Draw both carbocation intermediates that can be produced by the addition of a proton to the allene. (b) Explain the relative stabilities of those intermediates. Hint: Draw the orbital picture of the intermediates and consider whether the CH, groups in propadiene are in the same plane.
Treatment of propadiene (an allene) with hydrogen bromide produces 2-bromopropene as the major product. This suggests that the more stable carbocation intermediate is produced by the addition of a proton to Br HBr. H2C=C=CH, H3C CH2 a terminal carbon rather than to the central carbon. Propadiene 2-Bromopropene (a) Draw both carbocation intermediates that can be produced by the addition of a proton to the allene. (b) Explain the relative stabilities of those intermediates. Hint: Draw the orbital picture of the intermediates and consider whether the CH, groups in propadiene are in the same plane.
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 34MP: Reaction of iodoethane with CN- yields a small amount of isonitrile, CH3CH2N≡C, along with the...
Related questions
Question
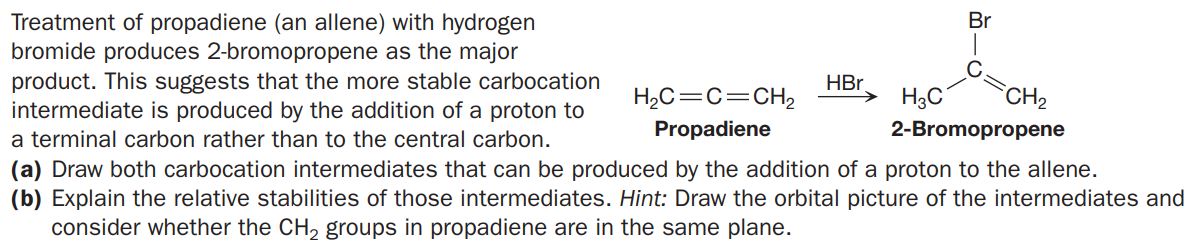
Transcribed Image Text:Treatment of propadiene (an allene) with hydrogen
bromide produces 2-bromopropene as the major
product. This suggests that the more stable carbocation
intermediate is produced by the addition of a proton to
Br
HBr.
H2C=C=CH,
H3C
CH2
a terminal carbon rather than to the central carbon.
Propadiene
2-Bromopropene
(a) Draw both carbocation intermediates that can be produced by the addition of a proton to the allene.
(b) Explain the relative stabilities of those intermediates. Hint: Draw the orbital picture of the intermediates and
consider whether the CH, groups in propadiene are in the same plane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning