TReview Topics) (References Use the References to access important vahues if needed for this question. For each bond, show the direction of polarity by selecting the correct partial charges. Ge-Se vGe-Br Y Br-Se The most polar bond is 9 more group attempts remaining Retry Entire Group Submit Answer hulu acer 96
TReview Topics) (References Use the References to access important vahues if needed for this question. For each bond, show the direction of polarity by selecting the correct partial charges. Ge-Se vGe-Br Y Br-Se The most polar bond is 9 more group attempts remaining Retry Entire Group Submit Answer hulu acer 96
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 15STP
Related questions
Question
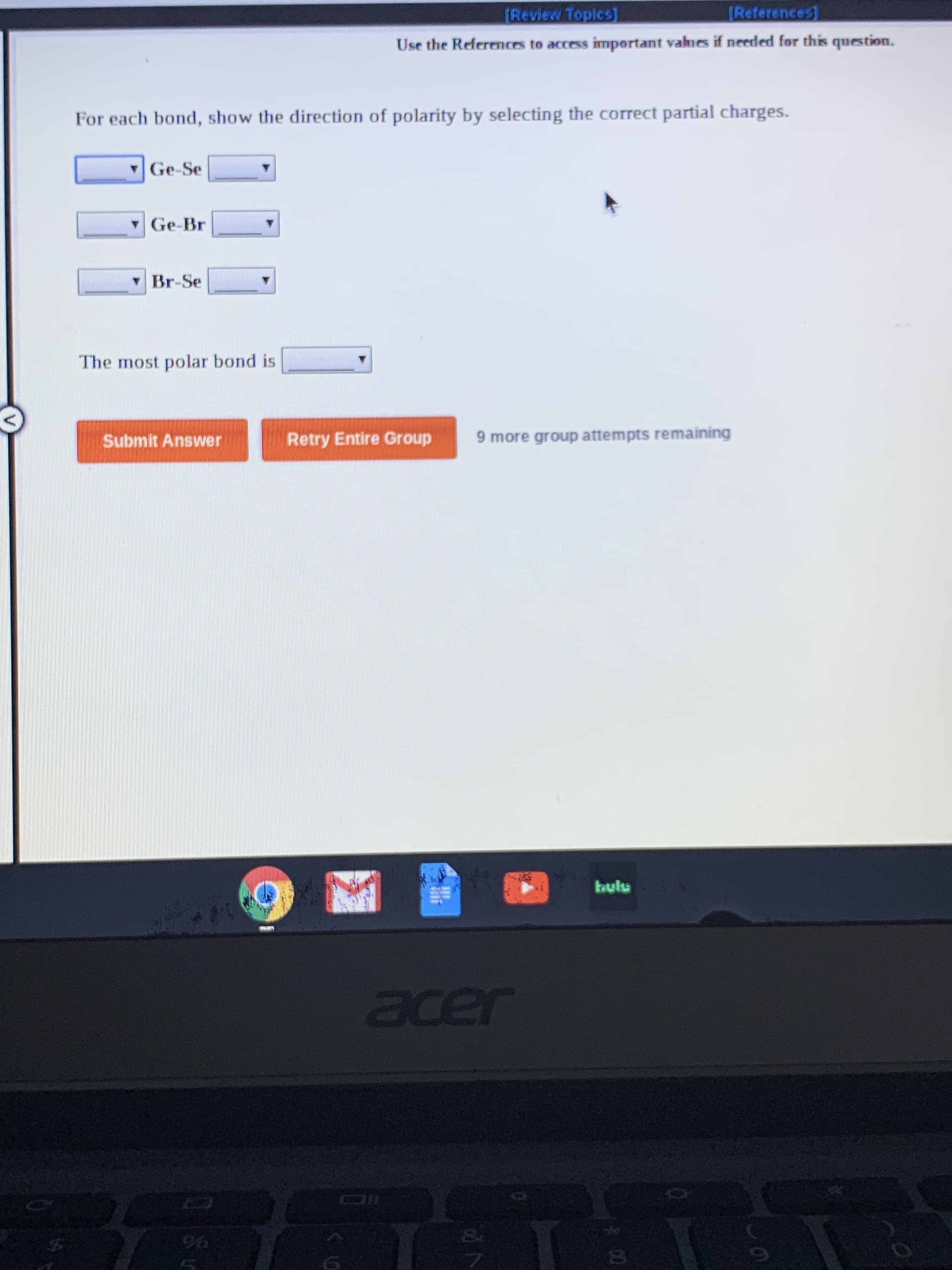
Transcribed Image Text:TReview Topics)
(References
Use the References to access important vahues if needed for this question.
For each bond, show the direction of polarity by selecting the correct partial charges.
Ge-Se
vGe-Br
Y Br-Se
The most polar bond is
9 more group attempts remaining
Retry Entire Group
Submit Answer
hulu
acer
96
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning