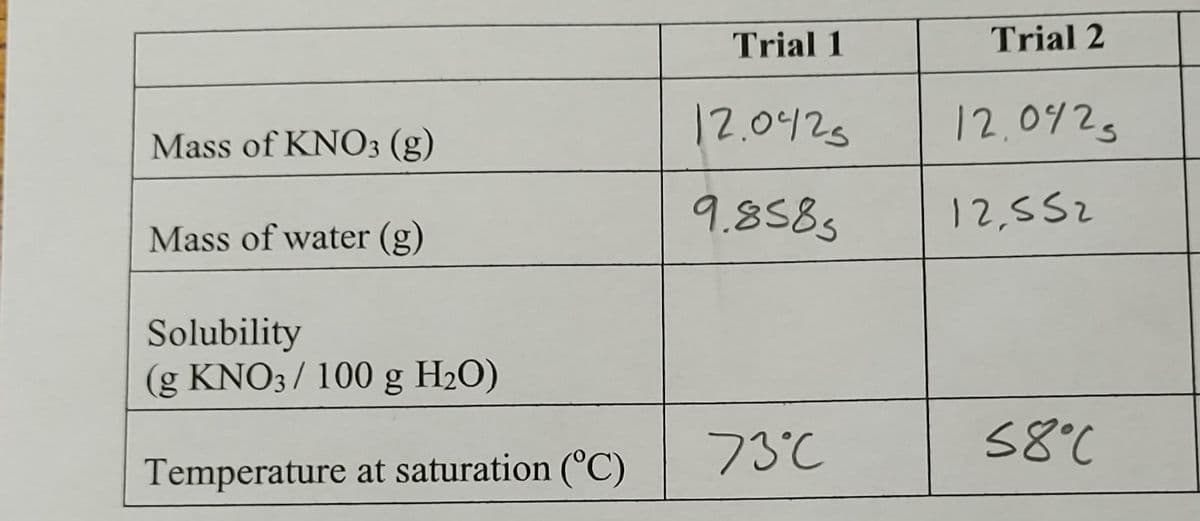
Trial 1 Trial 2 12.0425 12.042s Mass of KNO3 (g) 9.8585 12,552 Mass of water (g) Solubility (g KNO3/ 100 g H2O) 73°C 58°C Temperature at saturation (°C)
Q: Task 8. Eyo-Ball Method. An experiment to investigate the effect of temperature on the pressure of a…
A:
Q: Trial Trial Trial Trial 1.0 g 3.0 g 5.0 g 12.0g NaCi NaCI Naci Sucrose Mass of empty beaker (g)…
A:
Q: Mg Metal MgO Trial 1 Trial 2 Trial 1 Trial 2 Volume HCI 100 mL 100ML 0.2550g 58001 O.0276 moL Mass…
A: Given: Mg Metal MgO Volume of HCl 100 mL 100 mL Mass of solid Added 0.2550 g 1.008 g…
Q: es of CO₂ = (Mass of CO₂) ÷ (Molar Mass of CO₂) 0.781/44.01 = ar Volume = VSTP + Moles of CO₂ ent…
A: I think you have solved some of them correctly but mostly are wrong due to a minor mistake. Here, I…
Q: The 95 % confidence limit for Zn in certain water sample is 2.90± 0.25 mg/L. Circle the correct…
A:
Q: Solve for the Number of moles of the unknown with the given data. I do not no the given substance
A: Mass of the unknown, m = 0.5940 gPartial pressure of CO2 , P= 719.4 mm Hg = 719.4 / 760 = 0.9465…
Q: How many mL of a 0.250 M KCI solution must be diluted to 1.000 L so that the diluted solution…
A: Concentration of KCl stock solution = 0.250 M 1 M KCl contains 1M of K+. So, Concentration of K+…
Q: Model 2 – Experimental Data for Heating Water Experiment 1 Trial Mass (g) AT (°C) Added Energy…
A:
Q: Trial 1 Trial 2 Trial 3 Inital volume NaOH (mL) 50.00 50.00 50.00 Final volume NaOH (mL) 14.68 8.91…
A: Given: Initial volume of NaOH =I1 = 50.00mL Final volume of NaOH = F1 = 14.68mL Volume of NaOH used…
Q: Measurement Trial #1 Trial #2 Trial #3 mass of water/calorimeter/lid 82.73g 82.02g 84.46g…
A:
Q: Exp 4. Results Benzoic Acid Champi Sample Weight of sample 0.4997 g…
A: According to question, Benzoic Acid Champi Sample Weight of sample 0.4997 g 0.4107…
Q: 3-28 A 1.8-m3 rigid tank contains steam at 220°C. One-third of the volume is in the liquid phase and…
A: Introduction: In the above question two phases coexist in equilibrium ,thus we have a saturated…
Q: Data Table 1. Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Trial 6 Trial 7 Trial 8 Air 17.8 C 17.8 C 19.2…
A: The relation between density and volume is: d=mV Determination of no. of mol: n=wM Density of H2O2 =…
Q: DATA AND RESULTS A. Weight = 2.83 g B. Weight of water = 10 g C. Boiling point of distilled water =…
A: Solve the given question using ∆Tb formula which contains m, i, and Kb in it.
Q: What is the mass of air at 20 degree Celcius in a room with dimensions 3m by 4m by 5m? (P air = 1.20…
A:
Q: Calculate the density of water using the data below: * Weight of beaker and water, g 44.5369 Weight…
A: Given, Weight of beaker and water= 44.5369g Weight of beaker = 34.5802g Volume of water= 10mL We…
Q: Pre-lab: Experiment 6 All Sections 1. Define the following terms: Surface tension Viscosity Freezing…
A:
Q: How do I solve number 11?
A: The density of the substance can be described as the mass of the substance per unit volume. The…
Q: Calculations for experimentally determining R Trial 1 Trial 2 Mass of Mg ribbon (g) 0.031 0.039…
A: Trial 1: Given that, Mass of magnesium ribbon =0.031 g Temperature of H2(g) = 24.0 °C Volume of…
Q: Question attached
A: It is given that the molarity of NaOH solution is 0.100 M and volume of vinegar sample taken is 5…
Q: ANSWER IF DIRECTLY PROPORTIONAL, INDIRECTLY PROPORTIONAL, OR NO CORRELATION 1. Concentration of…
A: 1.croscarmellose sodium is used in pharmaceutical industries. It is used as disintegrant. Its…
Q: Sample answer format: -5.5 and 555.5 Using data from Table below, calculate the freezing and boiling…
A: First, calculate the molality of the solution: Molality = Mass of NaOHMolar mass of NaOH × 1000Mass…
Q: LOD 5D METHANOL CH40 D 4000 3347 8 3336 8 2945 18 2833 22 2522 77 2046 84 1460 47 3000 1116 62 1030…
A:
Q: M = wt (g) 1000 M. wt (B v (mL) mol 0.250 M: wt (g) 1000 58.4 () 1 (mL) wt (g) 0.0146 B mL %3D…
A:
Q: Trial 1 Trial 2 Trial 3 0.063g 0.063g 0.065g 1. Mass of Mg consumed, g 2. Moles of Mg consumed, n…
A:
Q: the injector is kept at higher temperature than the column so that it will move quickly. If the…
A: A multiple choice question based on analytical separations that is to be accomplished.
Q: Observations: Table 1: Estimation of DO. SI No Volume of water Initial sample (ml) burette Final…
A: The experiment is given as follows:
Q: Trial 1 Trial 2 0.063g 0.063g 1. Mass of Mg consumed, g 2. Moles of Mg consumed, n Oml 0ml 3.…
A: Eudiometer is a device used to measure the changes in in the volume of a gas in a reaction. It can…
Q: Writing a chemical equation from a molecular movie
A: Since in the picture we can see we have three types of molecules. 1) 2 white balls (i.e 2 H) and 2…
Q: a pharmacist added 15 g of 20% w/w calamine cream to 300g of aquaphor. what is the percent stregth…
A: The given data contains, mass of calamine =15 g of 20% =15×20100=3 gmass ofaquaphor= 300 gMass of…
Q: How much crystalline sugar (10% moisture content by weight) must be added to 35 litres of 10.5° Brix…
A: Volume of apple juice = 35 L…
Q: Trial 1 Trial 2 (Data Collected by (Data Collected by Group Member 1) Group Member 2) Concentration…
A:
Q: Standardization Data Table Trial 1 Trial 2 Trial 3 Mass KHP, g .422 .390 .475 Final Volume, mL 23.20…
A: The moles of a substance can be determined by dividing the mass of that substance by its molar mass.…
Q: 18 g of unknown organic sample was dissolve in 758 mL of Dicloromethane (DCM). The boiling point of…
A:
Q: 2-14. Estimate the critical temperature and critical pressure for a mixture of 80 weight percent…
A: Given:- Estimate the critical temperature and critical pressure for a mixture of 80 weight percent…
Q: IMOLK Data, Trial 2 Ideal Gas Law-PV=nRT Molarity of HCI (M): Data, Trial 1 3.00M 3.00 M Volume of…
A: Hii there, As there are multiple question posted. we are answering first question. If you need…
Q: 17 g of unknown organic sample was dissolve in 564 mL of Dicloromethane (DCM). The boiling point of…
A: NOTE: It will be benzene only and not DCM as the data is given for benzene and there is a typo error…
Q: Activity 2: Syrup Formula Sucrose ___ Purified water ___ To make 30ml…
A: The activity is about preparation of a syrup containing sucrose and water.
Q: Table 1. Room Temperature Raw Data Trial 1 Trial 2 Temperatue ("C) |21.5 °C |21.5 °C |Concentration…
A: During titration the solution of known molarity and volume taken in burette is called titrant. In…
Q: Example: Calculation the weight of barium iodate Ba(IO3)2 if it dissolved in 500mL of distilled…
A: Given data volume of solution = V = 500 mL = 0.500 L molar mass of Ba(IO3)2 = 487 g/mole Solubility…
Q: CAS No.: Solvent: BP: MP: .00 5 90 80 70 60 Reaction 3 50 40 30 20 10 7.0 6.0 2.0 1.0 00 5.0 No. 4.0…
A: NMR spectrum NMR spectrum of a compound gives the number of distinct protons present. When the…
Q: bblearn.cccnj.edu/webapps/assessment/take/launch.jsp?course assessmentuid- 29269 1&course id= 2992…
A: Given:Initial Volume V1 = 10.1 cm3 = 0.0101 L.Initial Pressure P1 = 746 mmHg = 0.9816 atm.Initial…
Q: Trial 1 Trial 2 1-Mass of empty evaporating dish 55.312 g 55.315 g 56.619 g 56.018 g 56.448 g 2-…
A: The mass of MgSO4 can be calculated by subtracting the mass of empty evaporating dish from the mass…
Q: DATA Table Trial 1 Trail 2 Trial 3 Mass cleaning solution (g) 10.1 10.1 9.8 Mass vinegar added to…
A: Acidity of vinegar solution = 5% Mass of acetic acid present in 100 g of vinegar solution = 5 g Mass…
Q: Part B. Standardization of Sodium hydroxide solution Balanced chemical equation: Trial 1 Trial 2…
A: Mass of Oxalic Acid 0.1314 g Initial burette reading 0.40 mL Final burette reading…
Q: chow Attempt History Current Attempt in Progress A dilute solution of hydrochloric acid with a mass…
A: Given: Mass of HCl=607.69g Mass of NaOH=610.71g
Q: The pressure in Denver, Colorado averages about 632 mm Hg. How many atmospheres is this? STARTING…
A:
Q: Question 1 a)Draw and explain the pressure -temperature diagram of wet gas reservoirs. b) The…
A: A gas reservoir is a naturally occurring storage area and characteristically a folded rock formation…
Q: the injector is kept at higher temperature than the column so that it will move quickly. If the…
A: In the given problem we have to check whether the given two statements are correct or not in…
How do you determine solubility for these two trials?

Step by step
Solved in 2 steps with 2 images

- Cyclohexanol 10 ml Mass of cyclohexanol 9.62 g – 0.053 mol Theroretical yield cyclohexene–7.89g - ? mol Therotical mass of cyclohexene- Actual yield- 6.8ml –4.055 g –? mol Percent yield- 51.04% Boiling point of cyclohexene -83 degree Celsius Theroretical boling point -83 degree Celsius can i get some clarification pls1.) Are there any intermolecular forces (IMF’s) between water molecules and cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of DHMIXING be for cyclohexane dissolving in water? a) Are there any IMF’s between water molecules and other water molecules? What kind(s)? Given this, what would the magnitude and sign of DHSOLVENT be for cyclohexane dissolving in water? c) Are there any IMF’s between cyclohexane molecules and other cyclohexane molecules? What kind(s)? Given this, what would the magnitude and sign of DHSOLUTE be for cyclohexane dissolving in water? d) Why do you think cyclohexane does not dissolve in water? Analyze this the way it was done in class, thinking about the three contributions to DHSOLUTION. What do you think the magnitude and sign of DHSOLUTION would be for dissolving cyclohexane in water? (Explain.) Would you expect these substances to dissolve in each other? Why?If 10.0mL of H2SO4 (specific gravity=1.50 containing 48.7% of combined SO3 by weight) is diluted to 400mL, what is the normality of the solution as an acid? 1.52 x 10-4 N 3.04 x 10-4 N 0.228 N 0.456 N
- What is the boiling point of the automobile radiator fluid prepared by mixing 1.02 L of ethylene glycol (HOCH2CH2OH, density = 1.114 g/mL) with 1.04 L of water (density = 1.000 g/mL)?Given the following mixture of two compounds 30.00 g of X (liquid) (MW =58.00 g/mol)(density 1.088 g/mL) and 880.00 g of Y (50.00 g/mol))(density 0.808 g/mL), calculate the mass percent of X. Given the following mixture of two compounds 35.00 mL of X (MW =80.00 g/mol)(density 0.945 g/mL) and 735.00 mL of Y (80.00 g/mol))(density 0.810 g/mL). The vapor pressure of pure Y is 47.00 torr. Calculate the vapor pressure of the solution.What is the boiling point of the automobile radiator fluid prepared by mixing 1.04 L of ethylene glycol (HOCH2CH2OH, density = 1.114 g/mL) with 1.08 L of water (density = 1.000 g/mL)?The Kb of water is 0.520°C/m.
- What is the boiling point of the automobile radiator fluid prepared by mixing 1.04 L of ethylene glycol (HOCH2CH2OH, density = 1.114 g/mL) with 1.08 L of water (density = 1.000 g/mL)? The Kb of water is 0.520°C/m.1a. If the mole fraction of cyclohexane in your mixture was 0.95, calculate the vapor pressure of your mixture if the vapor pressure of cyclohexane = 150.5 mm Hg. 1b. The vapor pressure of pure ethanol is 60.8 mm Hg. Would you expect it to have a higher boiling point or a lower boiling point that cyclohexane? Please answer part a and b and show work. Thanks3.1 Calculate the volume of concentrated (98% v.v) H2SO4 that you will need to prepare 250ml of an 5.4 M H2SO4 solution. Density of 98% v.v H2SO4 = 1.84 g/cm3
- One bottle of soda is stored in a refrigerator at 3 °C(276 K), and another is stored at room temperature (25 °C or 298 K). If both bottles are opened simultaneously, which one would exhibit greater carbonation(i.e., bubbles)? Explain.a) If a liquid with x(Ge)=0.4 is cooled, at what temperature does the first solid appear? b) What is the composition of this first solid? c) Describe the phases present at the eutectic temperature when a 50/50 mix of Al and Geat room temperature is heated. Be specific.What is the vapor pressure at 100 degree celsius of a solution containing 1.68 g of sucrose (MW 342.30) and 15.6 g of water (MW 18.2)?

