Trial Sample Mass Trial 1 Trial 2 (partner) (partner) #1374 o.1478018 0-lal NaOH Buret Readings Trial 1 Trial 2 Trial 3 Trial 4 35.10 mL 37.20mL Final mL 6.01 3.29 31.81 mL 29.9 ml 31.19. lo0 ml 6-o Initial ml mL Volume Delivered mL mL Volume NaOH (L) 00318)10.029910.631191 Moles NaOH Used Bol8Xole 2.99x mole 3.119x/0mole mo
Trial Sample Mass Trial 1 Trial 2 (partner) (partner) #1374 o.1478018 0-lal NaOH Buret Readings Trial 1 Trial 2 Trial 3 Trial 4 35.10 mL 37.20mL Final mL 6.01 3.29 31.81 mL 29.9 ml 31.19. lo0 ml 6-o Initial ml mL Volume Delivered mL mL Volume NaOH (L) 00318)10.029910.631191 Moles NaOH Used Bol8Xole 2.99x mole 3.119x/0mole mo
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 18E
Related questions
Question
100%
I've having trouble finding the average molar mass number and number of acidic hydrogens
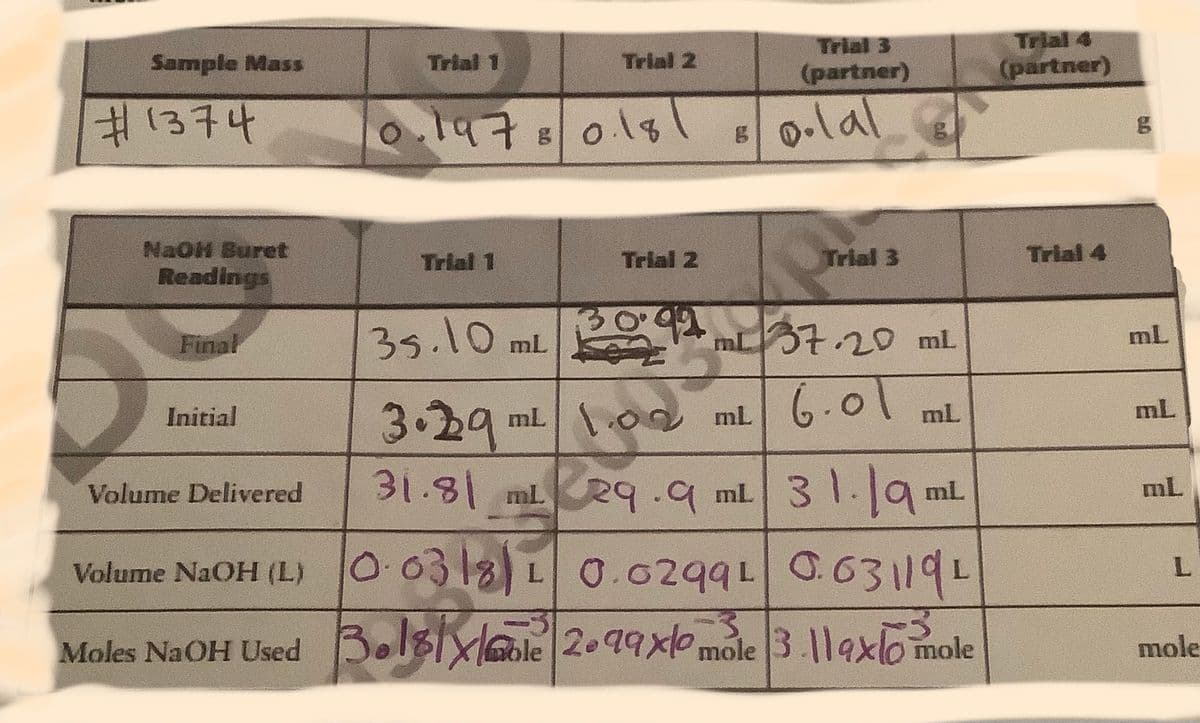
Transcribed Image Text:Trial 4
(partner)
Trial 3
Sample Mass
Trial 1
Trial 2
(partner)
#1374
o.1978018l
E0.lal
NaOH Buret
Readings
Trial 1
Trial 2
Trial 3
Trial 4
35.10 mL
mL37.20 mL
Final
mL
6.01
3.29"
31.81mLRq.9 ml 31.9 ml
mL
Initial
1.02 ml
mL
mL
31.19.
Volume Delivered
mL
Volume NaOH (L) 10.0318L0.0299L 0.63119L
Moles NaOH Used Bol8Ixlole 2.99x0mole 3.119xlomole
mole
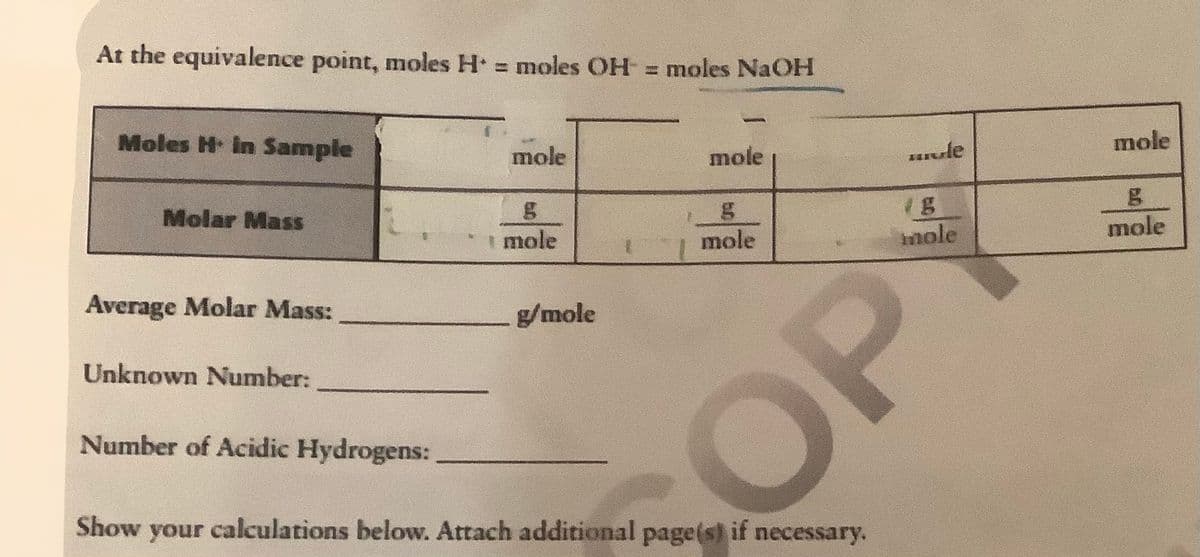
Transcribed Image Text:At the equivalence point, moles H = moles OH = moles NaOH
Moles H In Sample
mole
mole
vle
mole
Molar Mass
mole
mole
nole
mole
Average Molar Mass:
g/mole
Unknown Number:
Number of Acidic Hydrogens:
COP
Show your calculations below. Attach additional page(s) if necessary.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning