TV Watch Radian Majuts... O KINETICS AND EQUILIBRIUM Calculating the reaction rate of one reactant from that of another In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 619. liters per second of dinitrogen are consumed when the reaction is run at 216. °C and 0.17 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. x10 X Explanation Check O 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use SB II
TV Watch Radian Majuts... O KINETICS AND EQUILIBRIUM Calculating the reaction rate of one reactant from that of another In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 619. liters per second of dinitrogen are consumed when the reaction is run at 216. °C and 0.17 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. x10 X Explanation Check O 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use SB II
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 10E: In the PhET Reactions tab to observe how multiple atoms and molecules interact under varying...
Related questions
Question
Calculate the rate at which ammonium is being produced give your answers in kilogram per second round your answer to two significant digits.
Transcribed Image Text:TV Watch Radian
Majuts...
O KINETICS AND EQUILIBRIUM
Calculating the reaction rate of one reactant from that of another
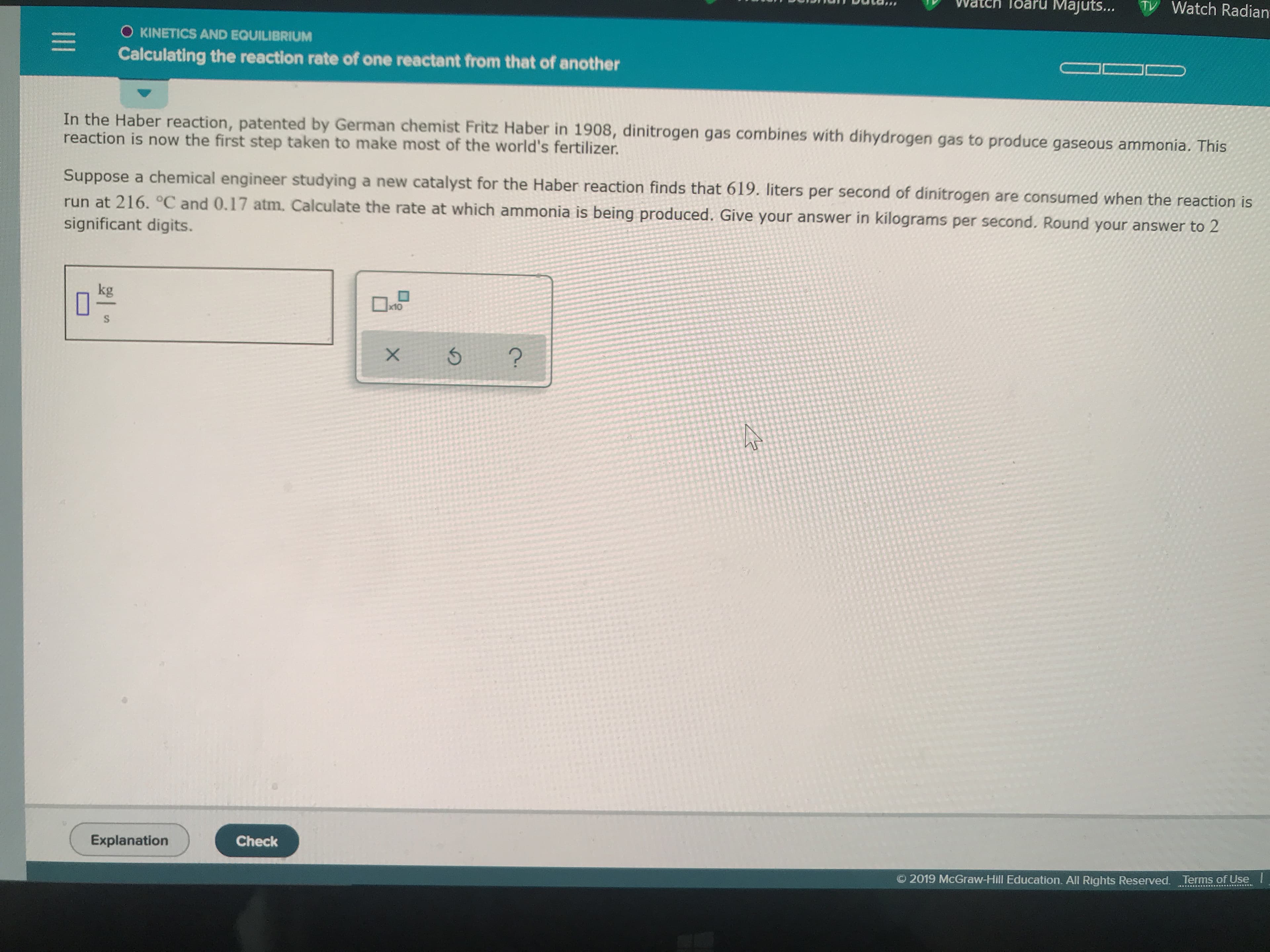
In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This
reaction is now the first step taken to make most of the world's fertilizer.
Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 619. liters per second of dinitrogen are consumed when the reaction is
run at 216. °C and 0.17 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2
significant digits.
x10
X
Explanation
Check
O 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use
SB
II
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,