Two rocks, rock one of heat capacity 15.67 JPC and rock two of heat capacity 37.93 JPC (note units!) are connected with a pipe of diamcter 0.139m and length 0.741m of a material that has a thermal conductivity of 116.13W/m°C. Initially rock one is at temperature 27.95°C and intially rock two is at 4.35°C. Write down the differential equation and solve it for the temperature of the rocks as a function of time, assuming that the pipe between them is always conducting heat as if it were in the quasi-equilibrium heat conduction (no transients in the pipe!) regime. (a) What is the final (infinite time) temperature of the system? (b) What is the temperature of rock 1 at time t=1.035 seconds. (c) At what time is rock two's temperature half the way to its final equilibrium (infinite time) temperature? (d) The entropy change of a rock is dS = dQ/T, which integrates to Delta S =C In(T/T,), where C is the heat capacity and T,T; are the final and initial temperatures respectively. Compute the total entropy change at infinito time of this system of two rocks. (a) °C C Answer part (a) (b) [ °C D Answer part (b) (c) s O Answer part (c)
Two rocks, rock one of heat capacity 15.67 JPC and rock two of heat capacity 37.93 JPC (note units!) are connected with a pipe of diamcter 0.139m and length 0.741m of a material that has a thermal conductivity of 116.13W/m°C. Initially rock one is at temperature 27.95°C and intially rock two is at 4.35°C. Write down the differential equation and solve it for the temperature of the rocks as a function of time, assuming that the pipe between them is always conducting heat as if it were in the quasi-equilibrium heat conduction (no transients in the pipe!) regime. (a) What is the final (infinite time) temperature of the system? (b) What is the temperature of rock 1 at time t=1.035 seconds. (c) At what time is rock two's temperature half the way to its final equilibrium (infinite time) temperature? (d) The entropy change of a rock is dS = dQ/T, which integrates to Delta S =C In(T/T,), where C is the heat capacity and T,T; are the final and initial temperatures respectively. Compute the total entropy change at infinito time of this system of two rocks. (a) °C C Answer part (a) (b) [ °C D Answer part (b) (c) s O Answer part (c)
Related questions
Question
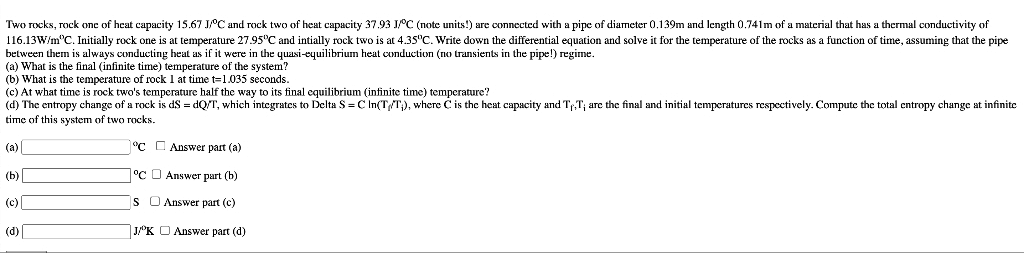
Transcribed Image Text:Two rocks, rock one of heat capacity 15.67 JPC and rock two of heat capacity 37.93 JC (note units!) are connected with a pipe of diameter 0.139m and length 0.741m of a material that has a thermal conductivity of
116.13W/m"C. Initially rock one is at temperature 27.95"C and intially rock two is at 4.35"C. Write down the differential equation and solve it for the temperature of the rocks as a function of time, assuming that the pipe
between them is always conducting heat as if it were in the quasi-equilibrium heat conduction (no transients in the pipe!) regime.
(a) What is the final (infinite time) temperature of the system?
(b) What is the temperature of rock 1 at time t=1.035 seconds.
(c) At what time is rock two's temperature half the way to its final equilibrium (infinite time) temperature?
(d) The entropy change of a rock is dS = dQ/T, which integrates to Delta S =C In(T/T;), where C is the heat capacity and Tr,T; are the final and initial temperatures respcctively. Compute the total entropy change at infinite
time of this system of two rocks.
(a)
"C C Answer part (a)
(b)
°C O Answer part (b)
(c)
Is O Answer part (c)
(d)
J/K O Answer part (d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps
