Types of Radioactive Decay Type of radiation Radiation emitted Example He-4 nucleus U-238 Th-234 alpha (He-4) Alpha Beta electron C-14N-14 + beta (e) high-energy photon Gamma Co-60 Ni-60 + y + e" The first entry in the table here shows the reaction that occurs when a U-238 isotope undergoes alpha decay. By how much do the atomic and mass numbers of the isotope change? Atomic number: -2 Mass number: 234 (1 of 6) Next Recheck 30th attempt
Types of Radioactive Decay Type of radiation Radiation emitted Example He-4 nucleus U-238 Th-234 alpha (He-4) Alpha Beta electron C-14N-14 + beta (e) high-energy photon Gamma Co-60 Ni-60 + y + e" The first entry in the table here shows the reaction that occurs when a U-238 isotope undergoes alpha decay. By how much do the atomic and mass numbers of the isotope change? Atomic number: -2 Mass number: 234 (1 of 6) Next Recheck 30th attempt
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section: Chapter Questions
Problem 9QAP
Related questions
Question
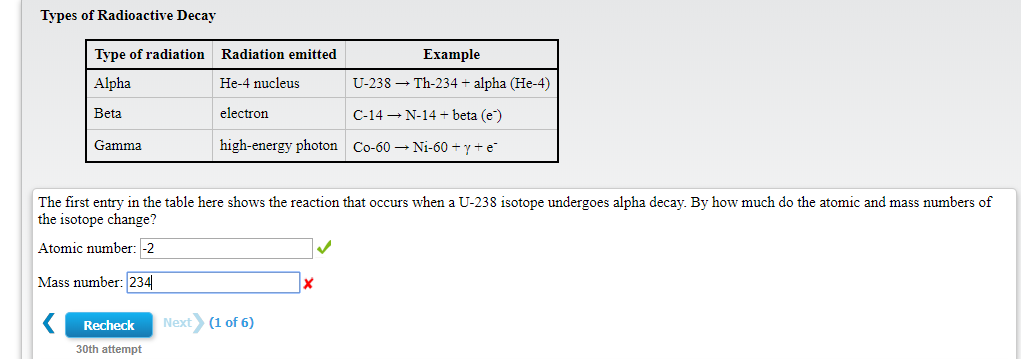
Transcribed Image Text:Types of Radioactive Decay
Type of radiation
Radiation emitted
Example
He-4 nucleus
U-238 Th-234 alpha (He-4)
Alpha
Beta
electron
C-14N-14 + beta (e)
high-energy photon
Gamma
Co-60
Ni-60 + y + e"
The first entry in the table here shows the reaction that occurs when a U-238 isotope undergoes alpha decay. By how much do the atomic and mass numbers of
the isotope change?
Atomic number: -2
Mass number: 234
(1 of 6)
Next
Recheck
30th attempt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning