Urea is an organic compound widely used as a fertilizer. Its solubility in water allows it to be made into aqueous fertilizer solutions and applied to crops in a spray. What is the maximum theoretical number of water molecules that one urea molecule can hydrogen bond with? Ignore shape for the purposes of this answer. Urea could theoretically form hydrogen bonds with this number of water molecules. Note, however, that the size and shape of a molecule may limit the number of hydrogen bonds formed by one urea molecule.
Urea is an organic compound widely used as a fertilizer. Its solubility in water allows it to be made into aqueous fertilizer solutions and applied to crops in a spray.
What is the maximum theoretical number of water molecules that one urea molecule can hydrogen bond with?
Ignore shape for the purposes of this answer.
Urea could theoretically form hydrogen bonds with this number of water molecules. Note, however, that the size and shape of a molecule may limit the number of hydrogen bonds formed by one urea molecule.
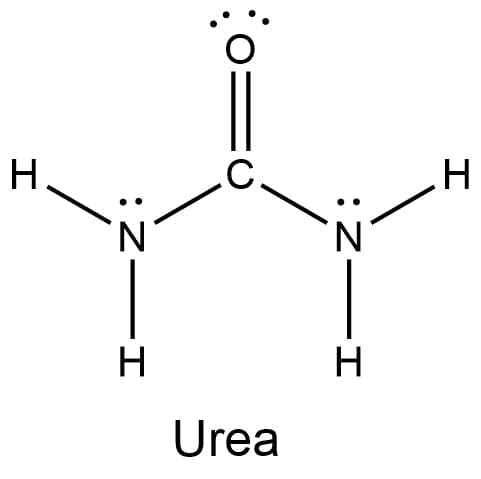
Urea, also recognized as carbamide, is an organic compound with a chemical composition of CO(NH2)2. This amide has two –NH2 groups joined by a functional carbonyl group. Urea plays an important function in the metabolism of animal nitrogen-containing compounds and is the primary nitrogen-containing material in mammalian urine. Urea is the primary nitrogen end product of protein metabolic degradation in both mammals and some fish. The substance is found not only in the urine of all animals, but also in their blood, bile, milk and perspiration.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









