Use the information and table to answer the question. A chemistry company is hired to produce sodium chloride (NaCl) using the following equation: Na (s) + HCI (ag) -> NaCl (aq) H2 (g) The team of chemists recorded the following data. Total Mass of Products Mass of Mass of Na HCI Solution 14.25 g 9.50 g 23.75 g How does the balanced chemical equation and data follow the law of conservation of matter? Explain
Use the information and table to answer the question. A chemistry company is hired to produce sodium chloride (NaCl) using the following equation: Na (s) + HCI (ag) -> NaCl (aq) H2 (g) The team of chemists recorded the following data. Total Mass of Products Mass of Mass of Na HCI Solution 14.25 g 9.50 g 23.75 g How does the balanced chemical equation and data follow the law of conservation of matter? Explain
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter7: Sollutions And Colloids
Section: Chapter Questions
Problem 7.2E
Related questions
Question
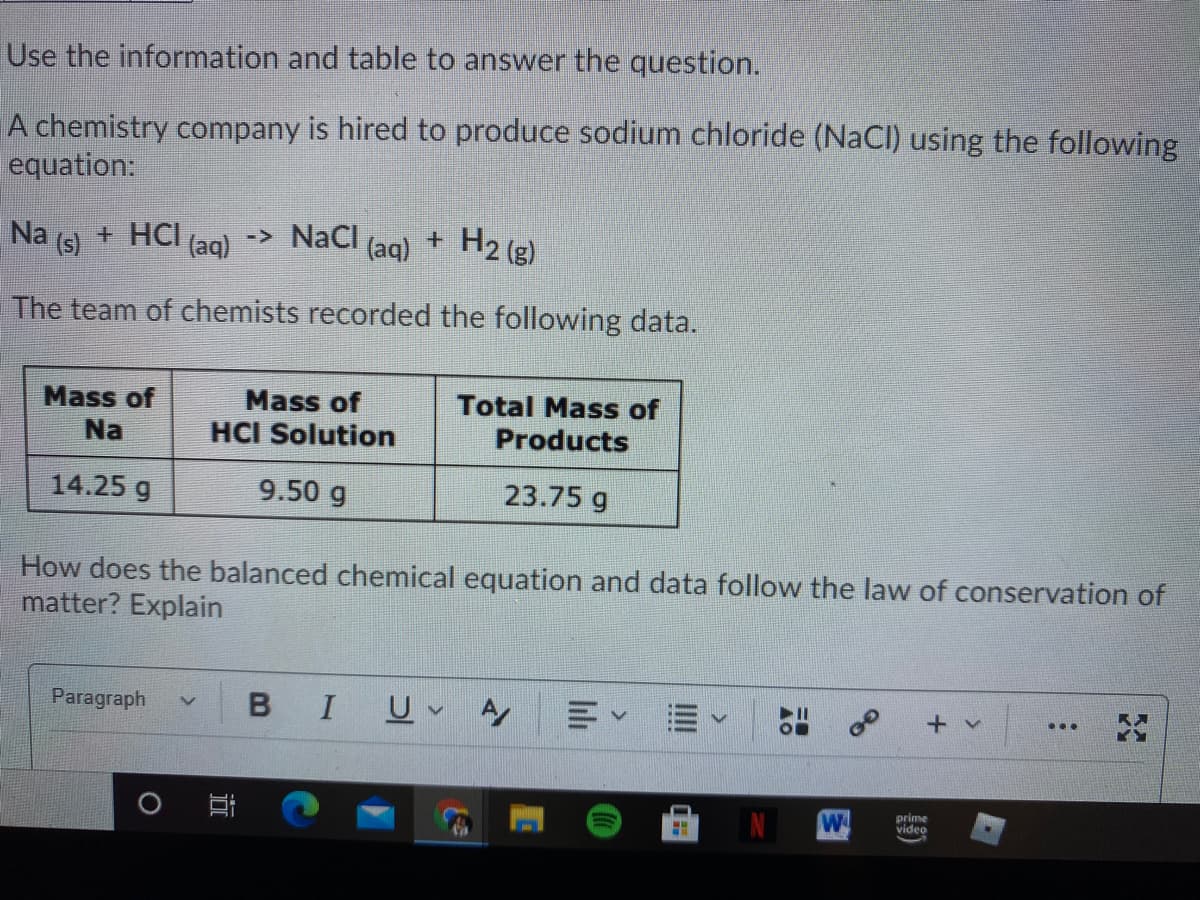
Transcribed Image Text:Use the information and table to answer the question.
A chemistry company is hired to produce sodium chloride (NaCI) using the following
equation:
Na
(s)
+ HCI (aq)
-> NaCl
(aq)
+ H2 (g)
The team of chemists recorded the following data.
Mass of
Mass of
Total Mass of
Na
HCI Solution
Products
14.25 g
9.50 g
23.75 g
How does the balanced chemical equation and data follow the law of conservation of
matter? Explain
Paragraph
I
+ v
...
W
prime
video
III
lılı
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning