Use the References to access important values if needed for this question. Write a balanced net ionic equation to show why the solubility of Zn(CN)2(s) increases in the presence of a strong acid and calculate the equilibrium constant Knet for the reaction of this sparingly soluble salt with acid. Be sure to specify states such as (aq) or (s). Knet Submit Answer Retry Entire Group 4 more group attempts remaining
Use the References to access important values if needed for this question. Write a balanced net ionic equation to show why the solubility of Zn(CN)2(s) increases in the presence of a strong acid and calculate the equilibrium constant Knet for the reaction of this sparingly soluble salt with acid. Be sure to specify states such as (aq) or (s). Knet Submit Answer Retry Entire Group 4 more group attempts remaining
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 1.3ACP: Use the formation constant of [Au(CN)2] in Appendix K to determine the equilibrium concentration of...
Related questions
Question
15b.
K net should be a number answer.
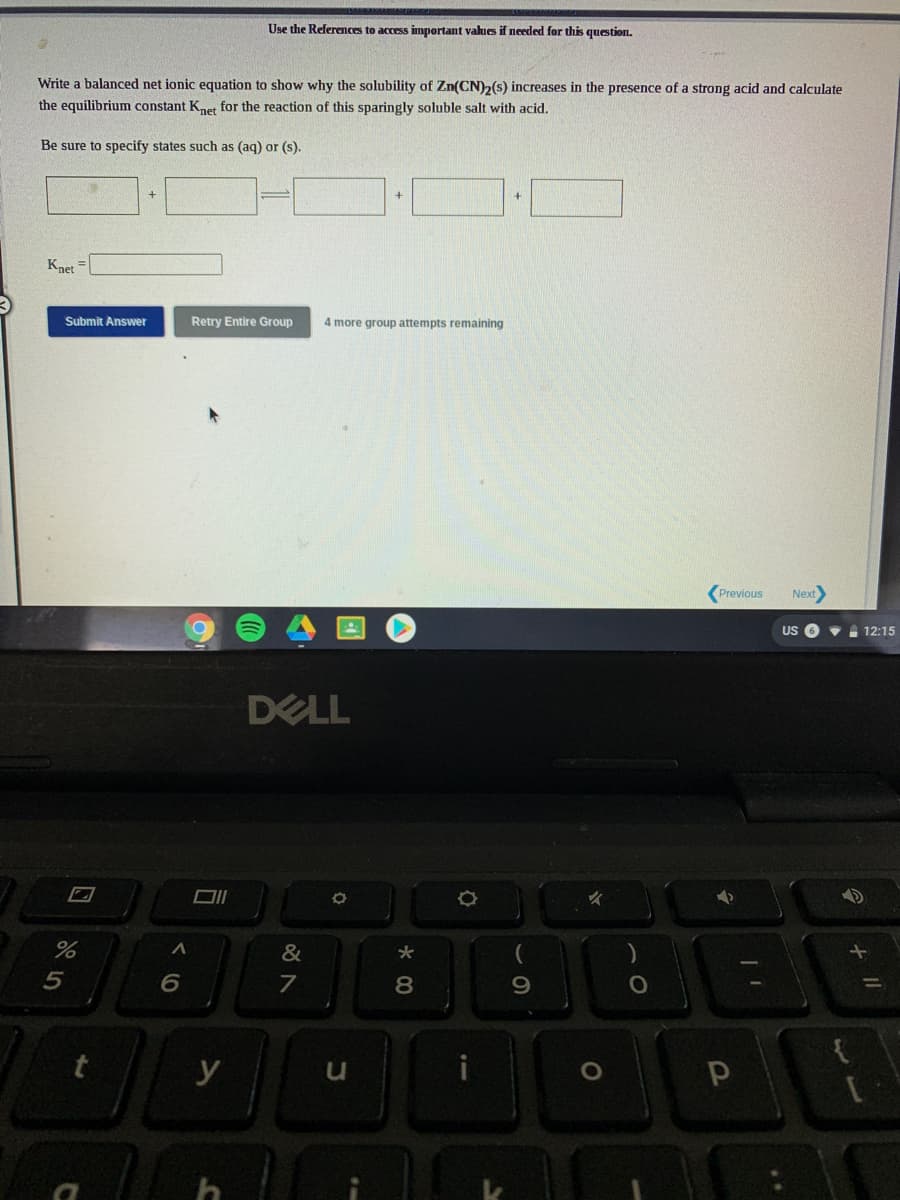
Transcribed Image Text:Use the References to access important values if needed for this question.
Write a balanced net ionic equation to show why the solubility of Zn(CN)2(s) increases in the presence of a strong acid and calculate
the equilibrium constant Knet for the reaction of this sparingly soluble salt with acid.
Be sure to specify states such as (aq) or (s).
Knet
Submit Answer
Retry Entire Group
4 more group attempts remaining
Previous
Next
US O • 12:15
DELL
&
5
8
9.
y
くO
口
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning