Use the solubility curve to help you answer the fol 150 KI 140 130 120 110 100 NANO3 90 80 70 60 NHACI KCI Naci 50 40 30 20 KCIO3. 10 Ce2(SO)3 0 10 20 30 40 50 60 70 80 90 100 Temperature (C) 1. Why does the x axis of the graph only go from 0°C to 100°C? 2. Which substance is most soluble at 10°C? 3. Which two substances share the same solubility at 80°C? Grams of solute XEHN' EON
Use the solubility curve to help you answer the fol 150 KI 140 130 120 110 100 NANO3 90 80 70 60 NHACI KCI Naci 50 40 30 20 KCIO3. 10 Ce2(SO)3 0 10 20 30 40 50 60 70 80 90 100 Temperature (C) 1. Why does the x axis of the graph only go from 0°C to 100°C? 2. Which substance is most soluble at 10°C? 3. Which two substances share the same solubility at 80°C? Grams of solute XEHN' EON
Question
100%
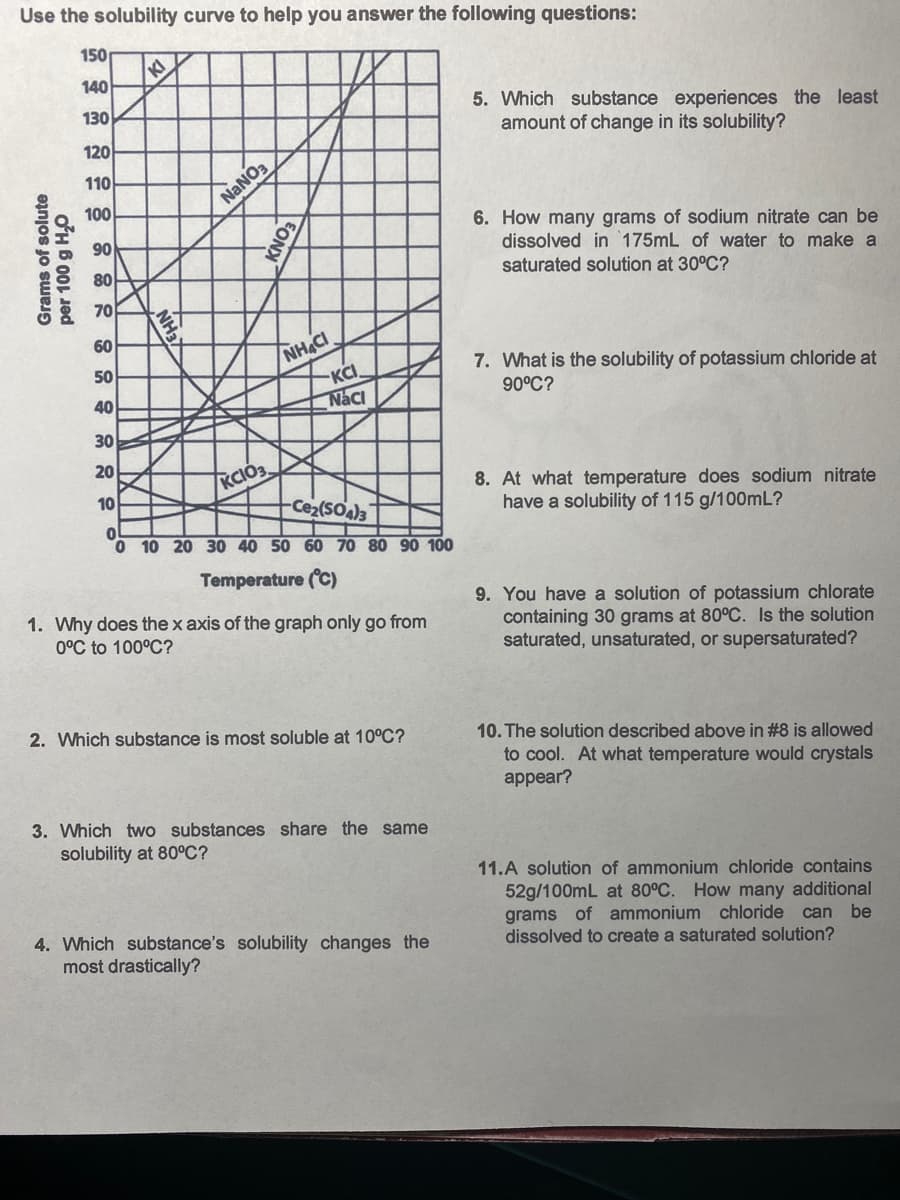
Transcribed Image Text:Use the solubility curve to help you answer the following questions:
150
140
5. Which substance experiences the least
amount of change in its solubility?
130
120
110
100
NANO3
6. How many grams of sodium nitrate can be
dissolved in 175mL of water to make a
saturated solution at 30°C?
90
80
70
NH&CI
KCI,
Naci
60
50
7. What is the solubility of potassium chloride at
90°C?
40
30
20
KCIO3
8. At what temperature does sodium nitrate
have a solubility of 115 g/100mL?
10
-Ce2(SO)3
0 10 20 30 40 50 60 70 80 90 100
Temperature (C)
1. Why does the x axis of the graph only go from
0°C to 100°C?
9. You have a solution of potassium chlorate
containing 30 grams at 80°C. Is the solution
saturated, unsaturated, or supersaturated?
2. Which substance is most soluble at 10°C?
10. The solution described above in #8 is allowed
to cool. At what temperature would crystals
appear?
3. Which two substances share the same
solubility at 80°C?
11.A solution of ammonium chloride contains
52g/100mL at 80°C. How many additional
grams of ammonium chloride can be
dissolved to create a saturated solution?
4. Which substance's solubility changes the
most drastically?
Grams of solute
per 100 g H,O
NH3
EONX
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.