Use the table 'Standard Reduction Potentials' located in the 'Tables', to predict if a reaction will occur between Cd metal and Br,1), when the two are brought in contact via half-cells in a voltaic cell. If a reaction will occur, write a balanced net ionic equation for the reaction, assuming that the product ions are in aqueous solution. If no reaction will occur, leave all boxes blank.
Use the table 'Standard Reduction Potentials' located in the 'Tables', to predict if a reaction will occur between Cd metal and Br,1), when the two are brought in contact via half-cells in a voltaic cell. If a reaction will occur, write a balanced net ionic equation for the reaction, assuming that the product ions are in aqueous solution. If no reaction will occur, leave all boxes blank.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 97QRT
Related questions
Question
100%
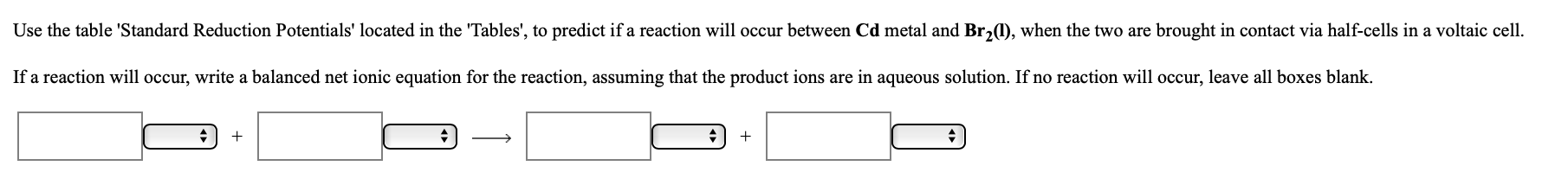
Transcribed Image Text:Use the table 'Standard Reduction Potentials' located in the 'Tables', to predict if a reaction will occur between Cd metal and Br,1), when the two are brought in contact via half-cells in a voltaic cell.
If a reaction will occur, write a balanced net ionic equation for the reaction, assuming that the product ions are in aqueous solution. If no reaction will occur, leave all boxes blank.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
