Use the your knowiedge of bonding and the molecular sructure to complete the following chan of properties for the molecules Carbon Tettachionde Hdrogen Chloride Ammonia Sodium Chloride Name Molecular Na: CI: Siructure Change in 21 Electronegativity Bond and Polanity Intermolecular Force
Use the your knowiedge of bonding and the molecular sructure to complete the following chan of properties for the molecules Carbon Tettachionde Hdrogen Chloride Ammonia Sodium Chloride Name Molecular Na: CI: Siructure Change in 21 Electronegativity Bond and Polanity Intermolecular Force
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 113AP: hy is the molecular structure of H2Ononlinear, whereas that of BeF2is linear, even though both...
Related questions
Question
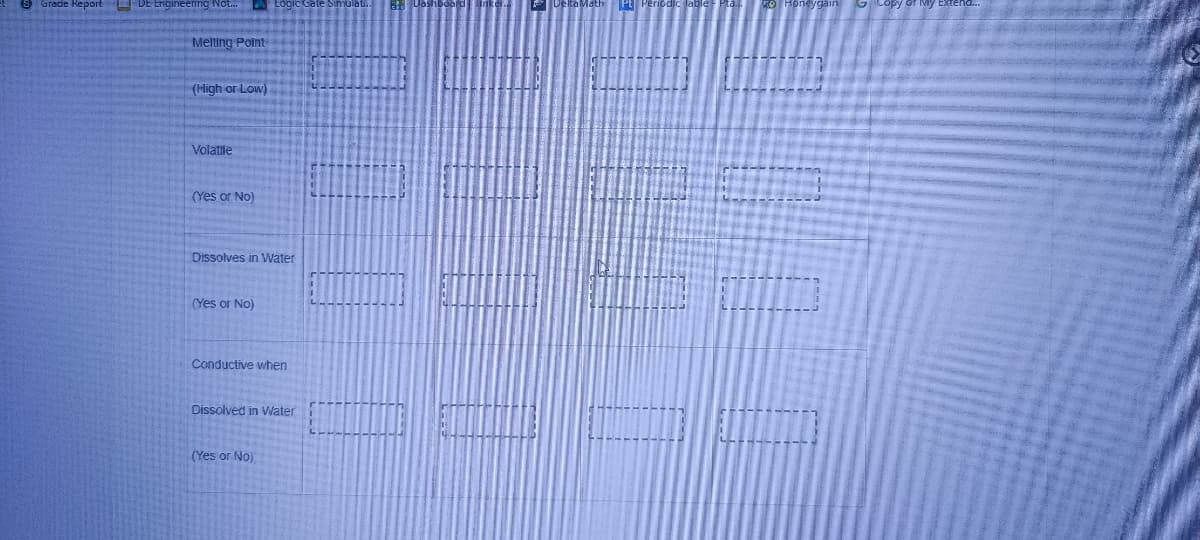
Transcribed Image Text:Grade Report DE Engineenng Not.
Logic Gate Sihulati.
Melting Point
(High or Low)
Volatile
(Yes or No)
Dissolves in VWater
(Yes or No)
Conductive when
Dissolved in VWater
(Yes or No)

Transcribed Image Text:Use the your knowledge of bofiding and the molecular structure to complete the following chart of properties for the molecules
Hvdrogen Chloride
Ammonia
Sodium Chloride
Name: Carbon Tettachloride
Molecular
Na: CI:
Structure
Change in
21
Electronegativity
Bond and
Polarity
Intermolecular
Force
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning