Used in everything from model planes to passenger planes, superglue is one of the best-known modern glues. The curing process that facilitates its remarkable (convert from liquid to solid) in the presence of adhesive properties is a chain polymerization reaction. The ingredient that gives superglue its adhesive ability is methyl cyanoacrylate. This compound is just one member of a larger family of cyanoacrylates with the following structure. Contrary to popular understanding, superglue does not"air dry."In fact, cyanoacrylates cure weak nucleophiles such as water. Under normal circumstances, a thin layer of water is present on almost all surfaces. The curing process, therefore, involves the reaction shown here. C=N C=N C=N C=N H,C=C H,O:+ H,C=C? H,O-CH,-C: + H,C= C=0 C=0 ČO,R C=0 R a cyanoacrylate R C=N C=N C=N - Hö-CH,- -C+H etc. HO-CH,-C-CH,-C: +H CO,R COOR ČOOR
Used in everything from model planes to passenger planes, superglue is one of the best-known modern glues. The curing process that facilitates its remarkable (convert from liquid to solid) in the presence of adhesive properties is a chain polymerization reaction. The ingredient that gives superglue its adhesive ability is methyl cyanoacrylate. This compound is just one member of a larger family of cyanoacrylates with the following structure. Contrary to popular understanding, superglue does not"air dry."In fact, cyanoacrylates cure weak nucleophiles such as water. Under normal circumstances, a thin layer of water is present on almost all surfaces. The curing process, therefore, involves the reaction shown here. C=N C=N C=N C=N H,C=C H,O:+ H,C=C? H,O-CH,-C: + H,C= C=0 C=0 ČO,R C=0 R a cyanoacrylate R C=N C=N C=N - Hö-CH,- -C+H etc. HO-CH,-C-CH,-C: +H CO,R COOR ČOOR
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter29: Organic Polymer Chemistry
Section: Chapter Questions
Problem 29.23P
Related questions
Question
Which of the following is not knowledge gained when one reads the name 2-octyl cyanoacrylate?
1. That the compound is anionic
2. That the compound would be chiral
3. That the compound is a nitrile
4. That the compound is an ester
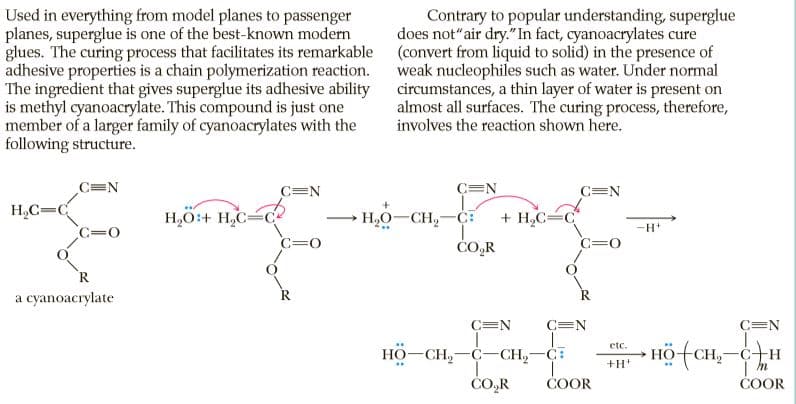
Transcribed Image Text:Used in everything from model planes to passenger
planes, superglue is one of the best-known modern
glues. The curing process that facilitates its remarkable (convert from liquid to solid) in the presence of
adhesive properties is a chain polymerization reaction.
The ingredient that gives superglue its adhesive ability
is methyl cyanoacrylate. This compound is just one
member of a larger family of cyanoacrylates with the
following structure.
Contrary to popular understanding, superglue
does not"air dry."In fact, cyanoacrylates cure
weak nucleophiles such as water. Under normal
circumstances, a thin layer of water is present on
almost all surfaces. The curing process, therefore,
involves the reaction shown here.
C=N
C=N
C=N
C=N
H,C=C
H,O:+ H,C=C?
H,O-CH,-C:
+ H,C=
C=0
C=0
ČO,R
C=0
R
a cyanoacrylate
R
C=N
C=N
C=N
- Hö-CH,-
-C+H
etc.
HO-CH,-C-CH,-C:
+H
CO,R
COOR
ČOOR
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning