Uses of Substances based on their Chemical Bonds Objective: To discover the uses of some substances based on their chemical bonds. I. I. Materials: Flashlight bulb 3V -battery Connecting wires (exposed ends) Metal strips of aluminum, copper, iron Distilled water NaCI Small Beaker II. Procedure: 1. To show the conductivity of table salt, NaCl, prepare a simple circuit consisting of a flashlight bulb, 3V- battery, and connecting wires with insulator ends removed. Prepare 200 cc of distilled water in a small beaker and immersed the ends of the connecting wire in the water as shown below. Observe the bulb if it would light. Mix 5 g of NaCl in the distilled water and observe the bulb. Did it light? electrodes wire saltwater battery light bulb 2. Add 5 g more, mix and observe the bulb again. 3. Repeat procedure 2. 4. To illustrate the electrical conductivity of different metals, prepare strips of aluminum, copper and iron. Use the circuit in procedure 1. Connect the bare ends of the wire to each of the metal strips and observe if the bulb will light. IV. Results: Check the appropriate observation. Conductivity of NaCl solution Bulb lights Bulb did not light Distilled water without NaCl Distilled water and 5g NaCl Distilled water and 10g NaCl Learning Module in Inorganic Chemistry Chapter 1: Laboratory Activities Distilled water and 15g NaCl Conductivity of Metal Strips Aluminum Copper Iron Guide questions: 1. Why did the bulb light when NaCl is mixed with distilled water? Explain in terms of chemical bonding. V. 2. Is there a difference in the bulb brightness for each of metal strip? Why? VI. Conclusion:
Uses of Substances based on their Chemical Bonds Objective: To discover the uses of some substances based on their chemical bonds. I. I. Materials: Flashlight bulb 3V -battery Connecting wires (exposed ends) Metal strips of aluminum, copper, iron Distilled water NaCI Small Beaker II. Procedure: 1. To show the conductivity of table salt, NaCl, prepare a simple circuit consisting of a flashlight bulb, 3V- battery, and connecting wires with insulator ends removed. Prepare 200 cc of distilled water in a small beaker and immersed the ends of the connecting wire in the water as shown below. Observe the bulb if it would light. Mix 5 g of NaCl in the distilled water and observe the bulb. Did it light? electrodes wire saltwater battery light bulb 2. Add 5 g more, mix and observe the bulb again. 3. Repeat procedure 2. 4. To illustrate the electrical conductivity of different metals, prepare strips of aluminum, copper and iron. Use the circuit in procedure 1. Connect the bare ends of the wire to each of the metal strips and observe if the bulb will light. IV. Results: Check the appropriate observation. Conductivity of NaCl solution Bulb lights Bulb did not light Distilled water without NaCl Distilled water and 5g NaCl Distilled water and 10g NaCl Learning Module in Inorganic Chemistry Chapter 1: Laboratory Activities Distilled water and 15g NaCl Conductivity of Metal Strips Aluminum Copper Iron Guide questions: 1. Why did the bulb light when NaCl is mixed with distilled water? Explain in terms of chemical bonding. V. 2. Is there a difference in the bulb brightness for each of metal strip? Why? VI. Conclusion:
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 83QRT
Related questions
Question
Transcribed Image Text:Uses of Substances based on their Chemical Bonds
Objective:
To discover the uses of some substances based on their chemical bonds.
I.
I.
Materials:
Flashlight bulb
3V -battery
Connecting wires (exposed ends)
Metal strips of aluminum, copper, iron
Distilled water
NaCI
Small Beaker
II.
Procedure:
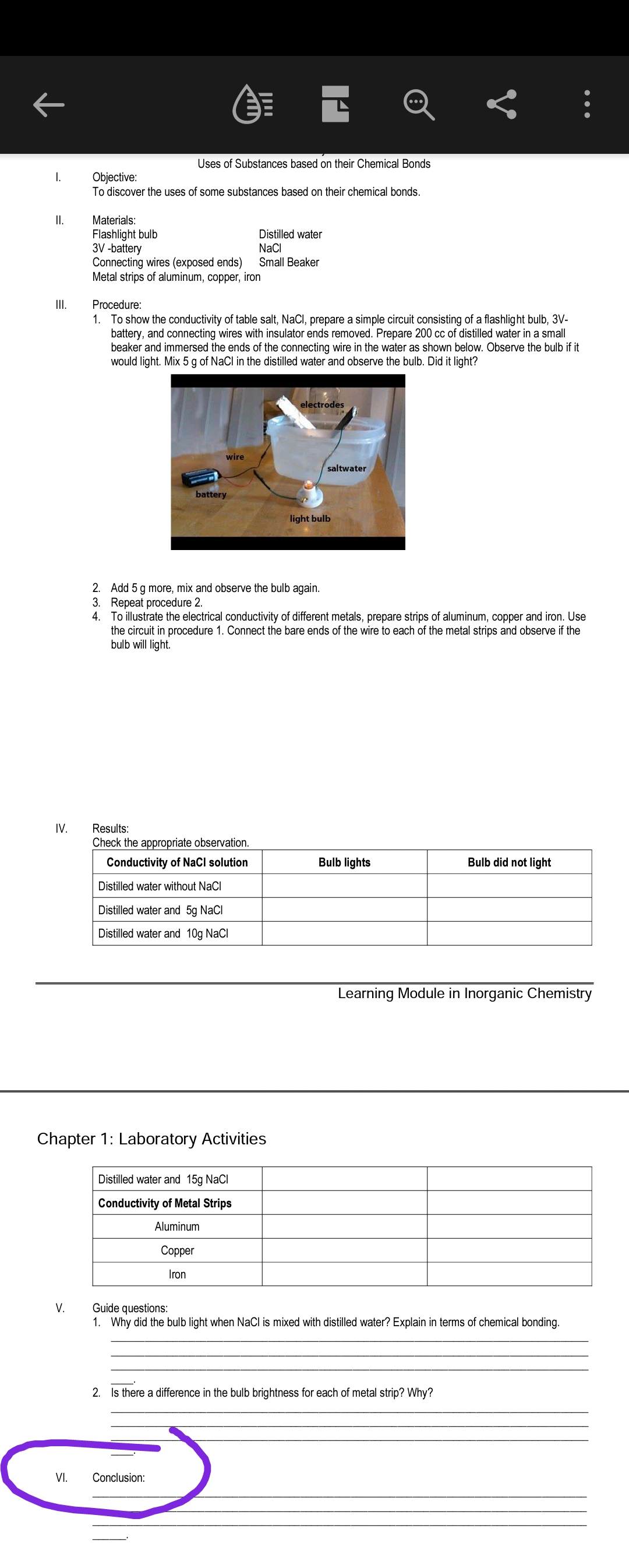
1. To show the conductivity of table salt, NaCl, prepare a simple circuit consisting of a flashlight bulb, 3V-
battery, and connecting wires with insulator ends removed. Prepare 200 cc of distilled water in a small
beaker and immersed the ends of the connecting wire in the water as shown below. Observe the bulb if it
would light. Mix 5 g of NaCl in the distilled water and observe the bulb. Did it light?
electrodes
wire
saltwater
battery
light bulb
2. Add 5 g more, mix and observe the bulb again.
3. Repeat procedure 2.
4. To illustrate the electrical conductivity of different metals, prepare strips of aluminum, copper and iron. Use
the circuit in procedure 1. Connect the bare ends of the wire to each of the metal strips and observe if the
bulb will light.
IV.
Results:
Check the appropriate observation.
Conductivity of NaCl solution
Bulb lights
Bulb did not light
Distilled water without NaCl
Distilled water and 5g NaCl
Distilled water and 10g NaCl
Learning Module in Inorganic Chemistry
Chapter 1: Laboratory Activities
Distilled water and 15g NaCl
Conductivity of Metal Strips
Aluminum
Сopper
Iron
Guide questions:
1. Why did the bulb light when NaCl is mixed with distilled water? Explain in terms of chemical bonding.
V.
2. Is there a difference in the bulb brightness for each of metal strip? Why?
VI.
Conclusion:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning