Using the Wijs Method, determine the Sample Weight of an unknown sample using the following data: lodine Number: 7.49 Titration of Blank Initial Burette Reading: 3.54 mL, Final Reading: 26.75 Titration of Sample Initial Reading: 10.38 mL, Final Reading: 21.43 mL
Using the Wijs Method, determine the Sample Weight of an unknown sample using the following data: lodine Number: 7.49 Titration of Blank Initial Burette Reading: 3.54 mL, Final Reading: 26.75 Titration of Sample Initial Reading: 10.38 mL, Final Reading: 21.43 mL
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter11: Solutions And Colloids
Section: Chapter Questions
Problem 50E: A sample of an organic compound (a nonelectrolyte) weighing 1.35 g lowered the freezing point of...
Related questions
Question
Whats the right choice
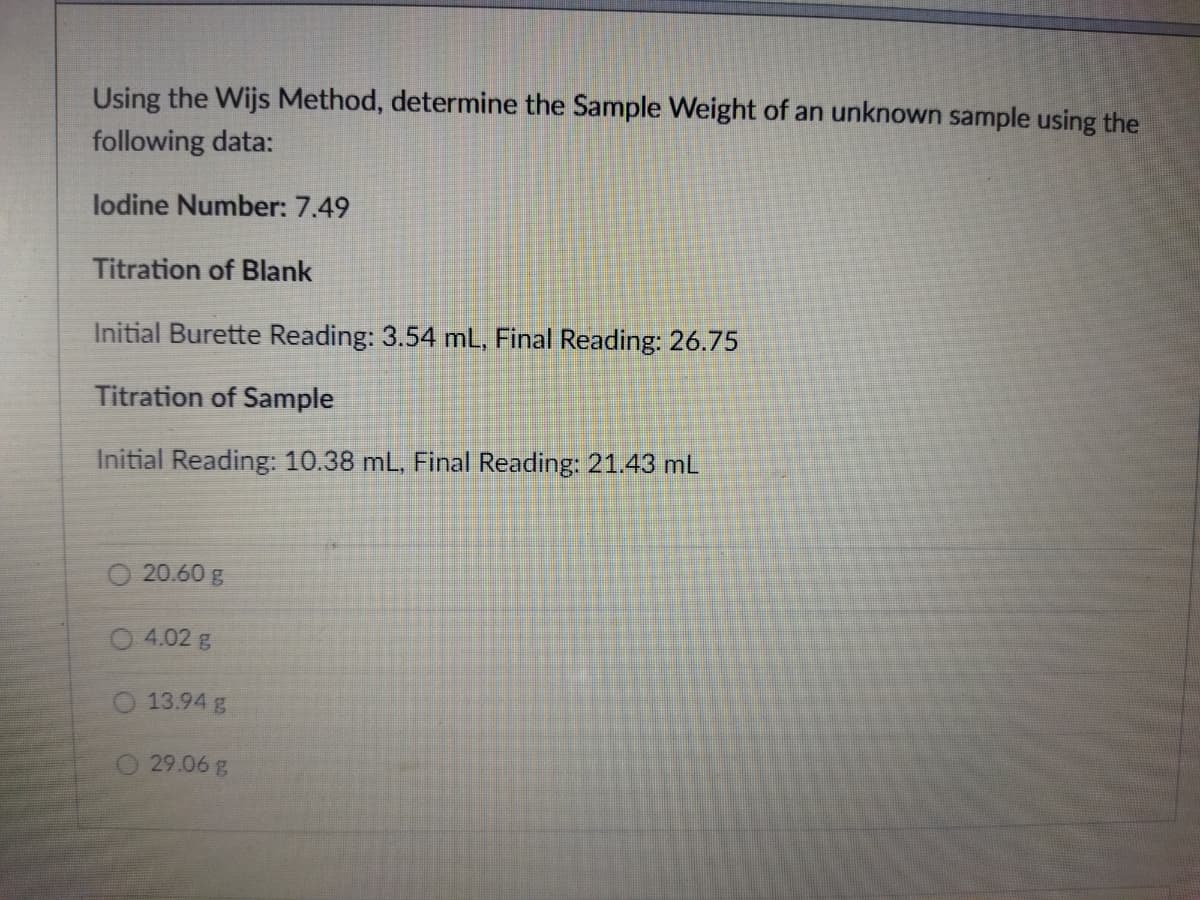
Transcribed Image Text:Using the Wijs Method, determine the Sample Weight of an unknown sample using the
following data:
lodine Number: 7.49
Titration of Blank
Initial Burette Reading: 3.54 mL, Final Reading: 26.75
Titration of Sample
Initial Reading: 10.38 mL, Final Reading: 21.43 mL
O 20.60 g
O 4.02 g
13.94 g
29.06 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
