V and K are constants that are > 0, explain why the units of K and V are μm and μm/s respectively?
V and K are constants that are > 0, explain why the units of K and V are μm and μm/s respectively?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
V and K are constants that are > 0, explain why the units of K and V are μm and μm/s respectively?
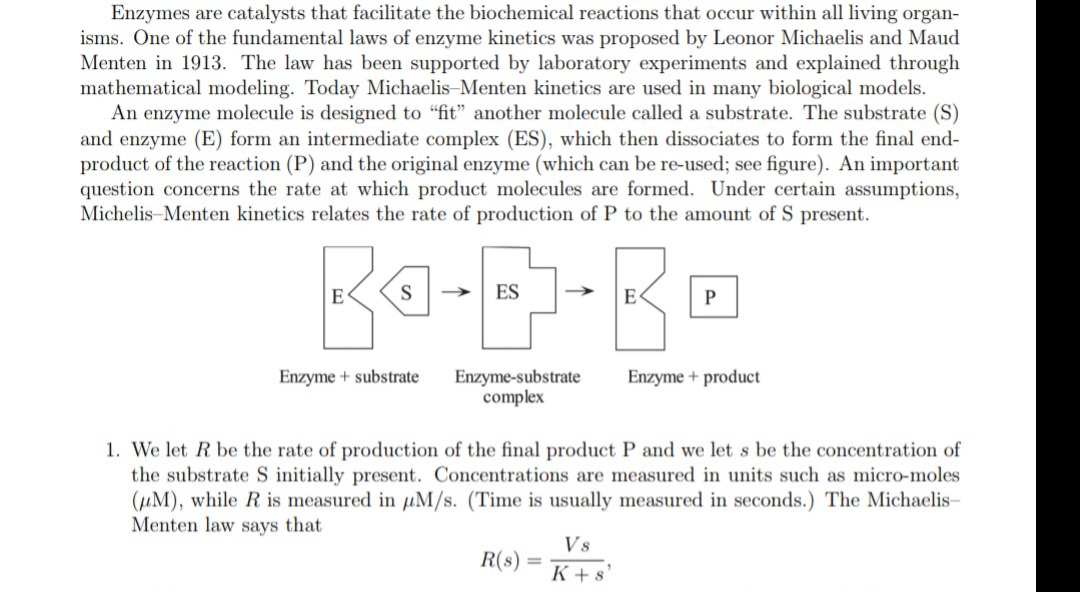
Transcribed Image Text:Enzymes are catalysts that facilitate the biochemical reactions that occur within all living organ-
isms. One of the fundamental laws of enzyme kinetics was proposed by Leonor Michaelis and Maud
Menten in 1913. The law has been supported by laboratory experiments and explained through
mathematical modeling. Today Michaelis-Menten kinetics are used in many biological models.
An enzyme molecule is designed to "fit" another molecule called a substrate. The substrate (S)
and enzyme (E) form an intermediate complex (ES), which then dissociates to form the final end-
product of the reaction (P) and the original enzyme (which can be re-used; see figure). An important
question concerns the rate at which product molecules are formed. Under certain assumptions,
Michelis-Menten kinetics relates the rate of production of P to the amount of S present.
S
ES
Enzyme-substrate
complex
Enzyme + substrate
Enzyme + product
1. We let R be the rate of production of the final product P and we let s be the concentration of
the substrate S initially present. Concentrations are measured in units such as micro-moles
(uM), while R is measured in uM/s. (Time is usually measured in seconds.) The Michaelis-
Menten law says that
Vs
R(s)
K+ s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON