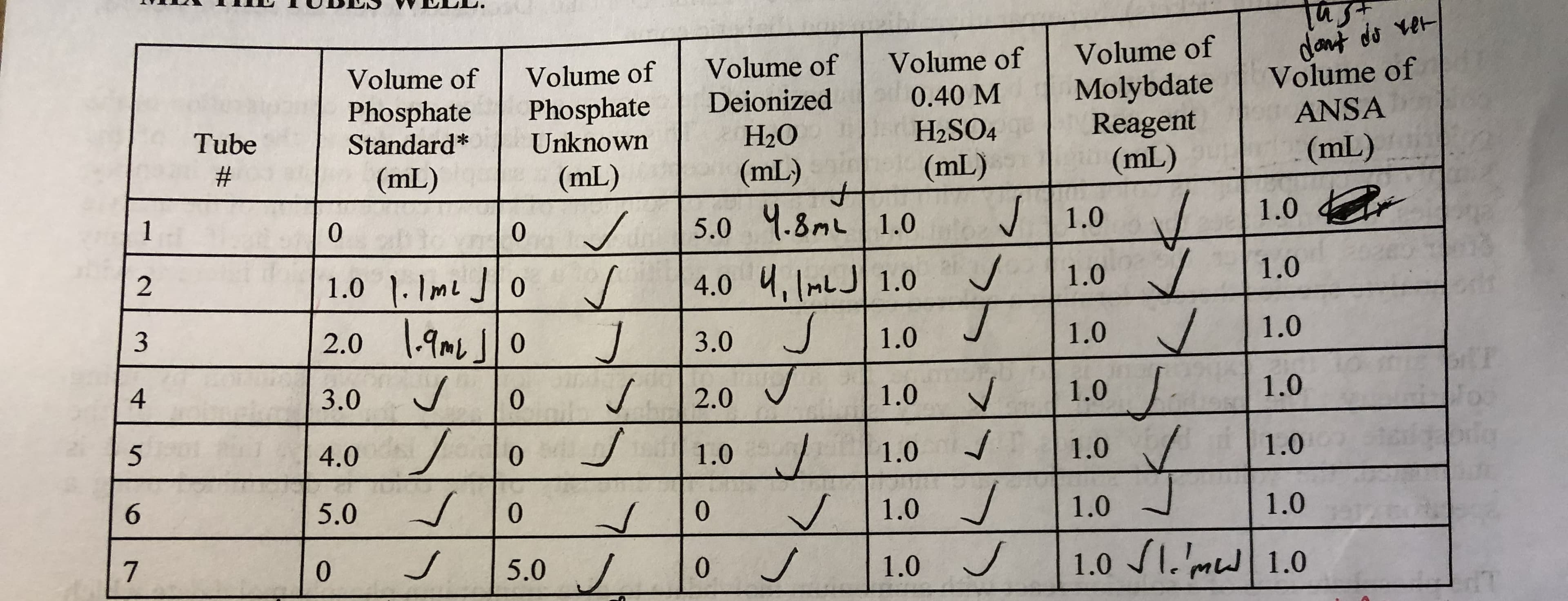
Volume of Volume of Volume of Volume of Volume of Phosphate | Phosphate | Deionized | 0.40M | Molybdate | Volume of H2SO4Reagent ANSA TubeStandardUnknown (mL) H2O (mL) (mL) 1.0 1.0 1.0 (mL) (mL) 1.0 5.0 Ч.3mLI 1.0 4.0 1.0 1.0 0 1.0 .Jo 1.0 4 3.0 4.0 5.0 0 2.0 1.0 1.0 1.0 0 1.0 1.0 1.0 o 5.0/
Q: How many mg/m3 is a 6% concentration of CO2. MW = C= 12 + O+O = 16+16 at NTP
A: 6% means 6 gm solvent in 100 ml solution
Q: The thiourea in a 1.563 g sample of an organic material was extracted into a dilute H2SO4 solution…
A: Given, Mass of thiourea = 1.563 g Molarity of Hg2+ = 0.009284 M Volume of Hg2+ = 36.43 mL Given…
Q: A solution is contaminated with traces of cadmium, manganese, and cobalt. Which option would be the…
A: One of the removal of heavy metals is by using the solubility factor of these metals. In a saturated…
Q: Hydroxyapatite, Ca,,(PO,),(OH), , has a solubility constant of Kp = 2.34 x 10–59, and dissociates…
A: Given Ksp of hydroxyapatite, Ca10(PO4)6(OH)2 , Ksp = 2.34*10-59 Given [OH-(aq)] = 5.30*10-4 M
Q: 2MNO, + 5C,0²- + 16H* → 2Mn²+ + 10 CO, + 8H,0 4. Here, 20 mL of 0.1 M KMNO, is equivalent to
A: Given:- 2MnO4 _ +5C2O42- +16 H+ -------->2Mn+2 +10CO2 + 8H2O. To find:- 20 mL of 0.1 M KMnO4…
Q: Disinfectant cleaner was the only substance that was entirely aqua Disinfectant Orange Juice…
A: NaHCO3 (sodium hydrogen carbonate) is baking soda and it is alkaline in nature whereas 3-5 % acetic…
Q: Multiple Choice Each of the numbered items or incomplete statements is followed by answers or by…
A: Titration is used to find the concentration of unknown solution by using the know concentration. In…
Q: Determine the percent yield of the synthesis. The % yield = 81.33 % The Theoretical Yield = 2.65g…
A: The percentage yield has to be given,
Q: Ascorbic acid (MW = 176.12 g/mole) is readily oxidized by molecular iodine in the presence of acid…
A: Oxidation reactions are those reactions in which oxidation number increases whereas reduction…
Q: Complete and balance the following redax equation. What is the coefficient of H20 when the equation…
A:
Q: . What are some precautions that should be observed when collecting samples for DO determination, to…
A: 1. precautions are - a) It is extremely important to prevent contamination of the sample with…
Q: A stock solution of 8uM dye is provided. While making one of the standard solution, a student…
A: To calculate the concentration of standard solution which is made from given stock solution of 8uM ,…
Q: A 2.432 grams of primary standard Na2CO3 (MW = 106) requires 47.26 mL of a H2SO4 solution to reach…
A: The given reaction is, CO32- + 2H+ ---> H2O + CO2 From the reaction, we can conclude that 1 mol…
Q: A chemical factory has been illegally disposing their chemical wastes without necessary…
A: Given, Site A B…
Q: Post-test 5 Q4 Directions: Determine if the following chemical reactions are oxidation-reduction…
A:
Q: In this experiment, your goal is to determine the amount of lead present in a water sample and, if…
A: Maximum allowed concentration of lead in drinking water are 0.01 mg/l and 0.015 mg/l. Amount of lead…
Q: In the acetic acid determination in vinegar experiment; NaOH was standardized with 0.25 g KHP (molar…
A:
Q: QUANTITY OF THERMOTOLERANT COLORFORM BACTERIA, WHICH IS ALLOWED IN 100 ML DRINKING WATER OF…
A: Option 4) lack of
Q: Lahen 9 5o1d is Impure, Its muting Point is higher and broader Tran the muhng unt fur pure Sampleã.…
A: When a pure substance is added with an impurities than the Properties like Melting point, boiling…
Q: Determine the solubility of Ag2SO3 (Ksp=1.5x10-14; MW=295.8 g/mole) in a 0.2 M solution of Na2SO3.
A:
Q: Identify the type of error (systematic or random) for each of the following situations below and…
A: Systematic error : it is repeatable error and consistent . it can be done by the analysts . it comes…
Q: 4. Calculate the cation exchange capacity of the soil in which the following amounts of exchangeable…
A: K+ = 0.0152 g = 0.0152 / 39.1 mole = 3.88 x 10-4 mol =…
Q: Q: The Soluibility of GaC13 is 0.004M. What is the Ksp? * O 7E-9 O 9 E-9 O 4.7 E-10 O 2.8 E-9 O Does…
A: The solubility product is simply defined as the product of the concentration of ions in a saturated…
Q: A mixture contains Na2CO3, NaOH, and inert matter. A sample weighing 1.500 g requires 28.85 mL 0f…
A:
Q: Mass of KxFe(C2O4)y · zH2O : 4.70 g Mass of sample : 0.175 g Mass of FeCl3 used in preparation :…
A: Step 1: In the given problem, a process of titration has to be done. In this method actually, the…
Q: Determine the concentration of 7.13 g zinc nitrate (MW = 189.4 g/mol) dissolved in 200.0 mL…
A: Given, Weight of zinc nitrate = 7.13 g Molecular weight (MW) of zinc nitrate = 189.4 g/mol Volume of…
Q: Calculate the volume of 0.4000M NaOH required to take the reaction of HCl+NaOH>H2O+NaCl to 70%…
A: In order to calculate the volume of NaOH required for the neutralization, we should calculate the…
Q: 56-57. What mass of Al(OH)3 would be produced if 350.0 mL of 0.4500 M KOH were added to a solution…
A: Here we are required to find the mass of aluminum hydroxide formed .
Q: QUESTION 17 The following are favorable precipitation conditions for the formation of large…
A: Precipitation is a process in solution the sagregation of molecules by intermolecular force of…
Q: An ore was found to contain iron and ferric oxide, if 0.5g of the ore, after solution in acid and…
A:
Q: An action will elevate the concentrations of three chemicals in the drinking water supply: 1,1,1-…
A: The concentration of these chemicals in drinking water is much higher than said to be safe for…
Q: In the Hoffman apparatus, the solution conducts electricity and water is decomposed to hydrogen at…
A: The Hoffman apparatus is used for the electrolysis of water. For electrolysis the water should be…
Q: Your body deals with excess nitrogen by excreting it in the form of urea, NH2CONH2. The reaction…
A: The number of moles of urea that equivalent to 95 mg of urea is; 1000 mg=1 gnurea=mureaMMurea=95…
Q: 25- How to prepare 70% (v/v) Ethanol in water? (Ethanol is absolute, Mwt 46 g/mol) A) By Diluting…
A: The concentration in v/v of some solute means the volume of solute that is present in a certain…
Q: In Module #4, we discussed the role of chlorofluorocarbons (CFCs) in catalyzing the destruction of…
A: Given data contains, Concentration of CF2Cl2 to decline is 0.22×10-11M. Rate constant is 0.009…
Q: What volume, in milliliters, of sodium acetate 1:150 (w/v) solution do you need to obtain 1.5 mEq…
A: The volume of sodium acetate solution (1:150 ; w/v) is 18.45 mL.
Q: After an excess of AgNO3 has been added to an aqueous solution containing dilute NaNO3 and KSCN,…
A: Ag has a strong affinity for thiocyanate ions,so when it is added to the aqueous solution of NaNO3…
Q: Convert the BOD concentration of 160 mg/L in the primary effluent into BOD loading rate in terms of…
A: The explanation is given below-
Q: 4. IH a gravimetric determination of sulfur as BaSO4, 0.8863 g of the ignited precipitate is found…
A: This problem requires basic analytical approach.
Q: A cork popper. To entertain children between the ages of 2 and 90, Daniel Harris enjoys popping…
A: Given: Formula mass of acetic acid = 60 g/mol Formula mass of sodium bicarbonate = 84 g/mol
Q: Write the methodology of this reaction Al + CuSO4 | Aluminum + Copper (II) Sulfate
A: methodology of this reaction => There are three steps involved in writing a chemical equation.…
Q: 500 g sampte containing only BaO (153.3 g/mol) and CaO (56.08 g/mol) was analyzed for its Ba and Ca…
A: Let the mass of barium oxide be x g and the mass of calcium oxide be (1.5 – x) g. When one mole of…
Q: Additives that may be included in the mobile phase for SEC hexane Salts Detergents Urea
A:
Q: . What is the gravimetric factor of SO3 in BaSO4? 2. What is the normality of an oxidizing agent of…
A: The question is based on the concept of analytical chemistry. We have to calculate gravimetric…
Q: What will be the ideal precipitating agent in gravimetric analysis? Select one: O React to all…
A: In this analytical technique, a precipitating agent is added to form precipitate of the analytical…
Q: Gravimetric analysis of Fe3O4 (MW = 232 g/mole) may be undertaken with the following reactions:…
A:
Q: a. An 18-mg strip of magnesium metal reacts in 5.0 mL of 3.0 M HCI over a given time period.…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Determine the volume of glacial acetic acid to prepare 500 ml of a 0.020 M acetic acid solution…
A:
Q: OPIC: GRAVIMETRY SHOW THE SOLUTION The mercury in a 0.7152-g sample was precipitated with an…
A: #Q.1: Given the mass of the impure sample = 0.7152 g Mass of Hg5(IO6)2(s) precipitate formed =…
how would I calculate the molarity ( in units of micromolar) of the phosphate of tube 7, with the molarity of phosphate being 250 micromoles/liter?
Step by step
Solved in 6 steps with 4 images

- Gravimetric analysis of Fe3O4 (MW = 232 g/mole) may be undertaken with the following reactions: Fe3O4 → Fe2O3 → Fe (OH)3. Weight of sample containing 8.00% Fe3O4 that must be taken to obtain a precipitate of Fe(OH)3 (MW = 107 g/mole) that weighs 150 mg is . a. 0.108 g b. 0.325 g c. 1.355 g d. 4.065 g Amount of Fe2O3 (MW = 160 g/mole) from which 150 mg of Fe(OH)3 (MW = 107 g/mole) may be obtained is . a. 0.112 g b. 0.224 g c. 0.448 g d. none of the other choicesA fermenter was filled with 10L of 0.6 mol/L sodium sulfite solution containing 0.003M Cu2+ ion and the air sparger was turned on. After exactly 10 minutes, the airflow was stopped and a 5 mL sample was taken and titrated. The concentration of sodium sulfite in the sample was found to be 0.2 M. Calculate the oxygen uptakePotassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.07
- A first-stage recovery of magnesium from seawater is precipitationof Mg1OH22 with CaO:Mg2+(aq) + CaO(s) + H2O(l)---->Mg(OH)2(s) + Ca2+(aq) What mass of CaO, in grams, is needed to precipitate 1000lb of Mg(OH)2?The solubility of borax, which is made up of sodium tetraborate (Na2B4O5(OH)4 8H2O), was analyzed. The dissolution of borax is: Na2B4O5(OH)4 • 8H2O(s) ⇌ 2 Na+(aq) + B4O5(OH)42–(aq) + 8 H2O(l) A 50 mL saturated solution was prepared. After filtration of solution, 5 mL aliquot was transferred to a flask and titrated using 0.432 M HCl. The endpoint was found to be 4.73 mL of the titrant. Tetraborate anion (B4O5(OH)42-) is a weak base which reacts with HCl like the following reaction: B4O5(OH)42–(aq) + 2 H+(aq) + 3 H2O(l) ⇌ 4 H3BO3(aq) What is Ksp expression for the dissolution? What is the tetraborate ions concentration in the filtrate? What is the molar solubility and Ksp of borax if the titration was done at room temperature (298 K)?Your body deals with excess nitrogen by excreting it in the form of urea, NH2CONH2. The reaction producing it is the combination of arginine (C6H14N4O2) with water to give urea and ornithine (C5H12N2O2). C6H14N4O2 + H2O ? NH2CONH2 + C5H12N2O2 [Molar masses: 174.2 18.02 60.06 132.2] If you excrete 95 mg of urea, what quantity of arginine must have been used? What quantity of ornithine must have been produced?
- To calculate the unknown concentration of a chemical in a solid sample, 4 different calibrationcurves were plot using 4 different methods. Which of the following method is considered asacceptable? a. Method 4, R? = 0.998b. Method 1, R2 = 0.650c. Method 2. R' = 0.890d. Method 3, R' = 0.169TOPIC: GRAVIMETRY SHOW THE SOLUTION The mercury in a 0.7152-g sample was precipitated with an excess of paraperiodic acid, H5IO6, according to the following reactions:5 Hg+2 + 2 H5IO6 ---> Hg5(IO6)2(s) + 10 H+The precipitate was filtered, washed free of precipitating agent, dried and found to weigh 0.3408-g. Calculate the percentage of Hg2Cl2 in the sample. Molar Masses: Hg5(IO6)2 = 1448.75 Hg2Cl2 = 472.09 Answer: 38.82% Hg2Cl2 An iron ore was analyzed by dissolving a 1.1324-g sample in concentrated HCl. The resulting solution was diluted with water, and the iron (III) was precipitated as the hydrous oxide Fe2O3·xH20 by the addition of NH3. After filtration and washing, the residue was ignited at a high temperature to give 0.5394 g of pure Fe2O3. Calculate (a) % Fe, and (b) % Fe3O4 in the sampleMolar Masses: Fe2O3 = 159.69 Fe = 55.847 Fe3O4 = 231.54 Answer: 33.32% Fe and 46.04% Fe3O4A Medical Technology student was given a capsule of a multivitamins and she was asked to determine the % by mass (w/w) of ascorbic acid present in the capsule. The student analyzed the 2.001 g sample using volumetric titration. The following data was generated in the analysis: KIO3 + 5KI + 6H+ → 3I2 + 6K+ + 3H2O C6H8O6 + I2 → C6H6O6 + 2I- + 2H+ Table 1. Standardization of KIO3 Molarity of Ascorbic Acid Standard Solution 0.03542 M Volume of Ascorbic Acid 25.00 mL Volume of KIO3 8.70 mL Molarity of KIO3 ______________M Table 2. Determination of Ascorbic Acid Concnetration Initial burette reading, KIO3 0.00 mL Final burette reading, KIO3 33.60 mL Volume consumed, KIO3 33.60 mL MM of Ascorbic Acid 176.12 g/mole choices 7.30% 30.1% 33.6% 32.5%
- A weight of 0.50 g was taken impure container containing sodium carbonate and bicarbonate. Dissolved in water and then crushed with hydrochloric acid (0.1 N), the burette reading game was at the endpoint of phenolphthalein of 10.5 ml and at the end point of the orange methylation point 30.1 ml. The percentage of sodium carbonate was in ................. knowing that the weights are: Na: 23, C: 12, O: 16An impure sample of calcium carbonate with a mass of 7.95 g was reacted with 50.00 cm3 of 1.00 mol dm hydrochloric acid (an excess). The resulling solution was transferred to a volumetric flask and titrated with 11.10cm3 of 0.300 mol dm-3 sodium hydroxide solution. Determine the percentage purity by mass of the calcium carbonate sample.CaCO3 + HCl -> CaCl2 + H2O + CO2 HCl + NaOH -> NaCl +H2O a. Determine how many moles of hydrochloric acid were used.b. Determine how many moles of excess HCI was titratedc. Determine how much in moles calcium carbonate present in the sample.d. Calculate the mass of calcium carbonate presente. Determine the percentwge calcium carbonate is in the sample.1,5419 g of magnetite (Fe3O4) ore; in concentrated HCL to form a mixture of Fe + 2 and Fe+3it's unraveling. By adding HNO3 to it, all Fe+2 s are upgraded to Fe+3. And the addition of FE+3 s NH3,with Fe (OH)3, precipitating into. The sediment is weighed as 0.8525 g in the form of Fe2O3 after the necessary operations. Calculate the percentage of Fe3O4 in the sample.

