Volume %=Volume of Solute / Volume of Solution ×100% I used 1/2 cup of granulated sugar and 1/2 tsp of the drink mix, and 3 cups of water. 2. Calculate the concentration of each diluted solution using the equation below and the volumes in Table. C1V1=C2V2 Where: C1=concentration of stock solution V1=volume of stock solution C2=concentration of diluted solution V2=volume of diluted solution (This is all the information that I have)
Volume %=Volume of Solute / Volume of Solution ×100% I used 1/2 cup of granulated sugar and 1/2 tsp of the drink mix, and 3 cups of water. 2. Calculate the concentration of each diluted solution using the equation below and the volumes in Table. C1V1=C2V2 Where: C1=concentration of stock solution V1=volume of stock solution C2=concentration of diluted solution V2=volume of diluted solution (This is all the information that I have)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section12.1: Characteristics Of Chemical Equilibrium
Problem 12.1E: The introduction to this chapter states that at a given temperature the concentration of a pure...
Related questions
Question
100%
- Calculate the concentration of sugar in this solution using the volume percent equation. For the purpose of this exercise, it can be assumed that the weight of the drink mix is 0.00 g. The volume of the solution is 3 and 1/3 cups. Calculate and record the volume percent (g/mL). (Note that 1 cup = 236 mL).
Volume %=Volume of Solute / Volume of Solution ×100%
I used 1/2 cup of granulated sugar and 1/2 tsp of the drink mix, and 3 cups of water.
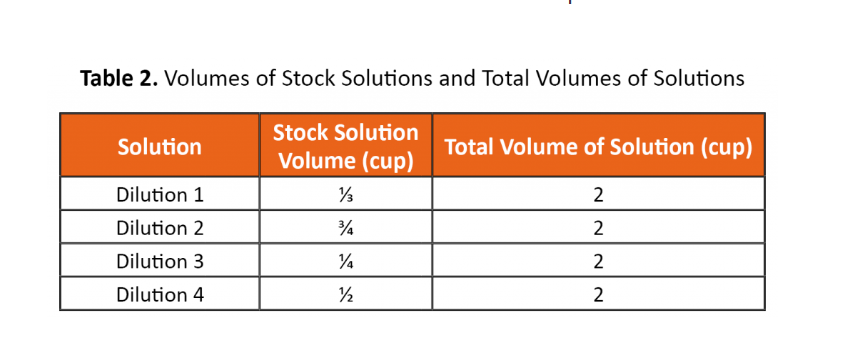
2. Calculate the concentration of each diluted solution using the equation below and the volumes in Table.
C1V1=C2V2
Where:
C1=concentration of stock solution
V1=volume of stock solution
C2=concentration of diluted solution
V2=volume of diluted solution
(This is all the information that I have)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning