Water flowing at a rate of 0.667 kg/s enters a countercurrent heat exchanger at 308 K and is heated by an oil stream entering at 383 K at a rate of 2.85 kg/s (Cp = 1.89 kJ/kg - K). The overall U = 300 W/m2K and the area A = 15.0 m2. Calculate the heat-transfer rate and the exit-water temperature. Instead of using 370 K for our assumption, please use 361 K as the value for Tco. Find the new: 1. Tc ave where: Tc ave = 0.5(Tco+Tci) 2. Cpc at Tc ave 3. Cmin 4. NTU 5. € 6.q 7. Tco
Water flowing at a rate of 0.667 kg/s enters a countercurrent heat exchanger at 308 K and is heated by an oil stream entering at 383 K at a rate of 2.85 kg/s (Cp = 1.89 kJ/kg - K). The overall U = 300 W/m2K and the area A = 15.0 m2. Calculate the heat-transfer rate and the exit-water temperature. Instead of using 370 K for our assumption, please use 361 K as the value for Tco. Find the new: 1. Tc ave where: Tc ave = 0.5(Tco+Tci) 2. Cpc at Tc ave 3. Cmin 4. NTU 5. € 6.q 7. Tco
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
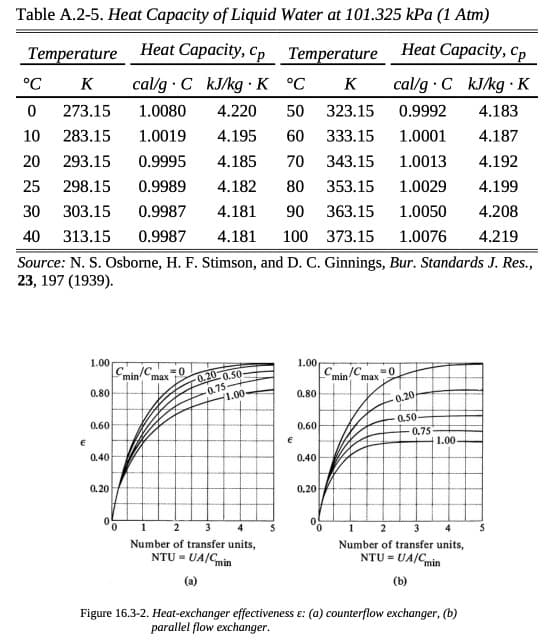
Transcribed Image Text:Table A.2-5. Heat Capacity of Liquid Water at 101.325 kPa (1 Atm)
Heat Capacity, Cp
.
cal/g C kJ/kg K
Temperature
°C
K
0 273.15
10 283.15 1.0019
20 293.15 0.9995
25 298.15 0.9989
30
303.15
0.9987
40 313.15 0.9987 4.181 100
Source: N. S. Osborne, H. F. Stimson, and D. C. Ginnings, Bur. Standards J. Res.,
23, 197 (1939).
€
1.00
0.80
0.60
0.40
0.20
Heat Capacity, Cp
Temperature
cal/g C kJ/kg K °C
K
323.15
0.9992
4.183
1.0080 4.220 50
4.195
60
333.15
1.0001
4.187
4.185
70 343.15
1.0013
4.192
4.182 80 353.15 1.0029
4.199
4.181 90 363.15
1.0050
4.208
373.15 1.0076 4.219
C /C =0
min max 0.20 0.50-
-0.75
1.00-
0
1
2
3
Number of transfer units,
NTU = UA/Cmin
€
1.00,
0.80
0.60
0.40
0,20
0
C
JC. =0
min max
0
0.20
0.50-
0.75
1.00-
1 2 3 4
Number of transfer units,
NTU = UA/C min
(b)
Figure 16.3-2. Heat-exchanger effectiveness &: (a) counterflow exchanger, (b)
parallel flow exchanger.
5

Transcribed Image Text:Question:
Water flowing at a rate of 0.667 kg/s enters a countercurrent heat exchanger at 308 K and is heated by an oil stream entering at 383 K
at a rate of 2.85 kg/s (Cp = 1.89 kJ/kg K). The overall U = 300 W/m2 K and the area A = 15.0 m2. Calculate the heat-transfer rate and
the exit-water temperature.
Instead of using 370 K for our assumption, please use 361 K as the value for Tco.
Find the new:
1. Tc ave where: Tc ave = 0.5(Tco+Tci)
2. Cpc at Tc ave
3. Cmin
4. NTU
5. €
6. q
7. Tco
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The