What are the best reagents to perform this transformation? ? ОН Он н РСС, CH2CI2 CrO3, H30, acetone OKMnO4, Na OH, cold enter your search term Na2Cr207, H2SO4, H20
What are the best reagents to perform this transformation? ? ОН Он н РСС, CH2CI2 CrO3, H30, acetone OKMnO4, Na OH, cold enter your search term Na2Cr207, H2SO4, H20
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter16: Synthesis Workshop 1
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
100%
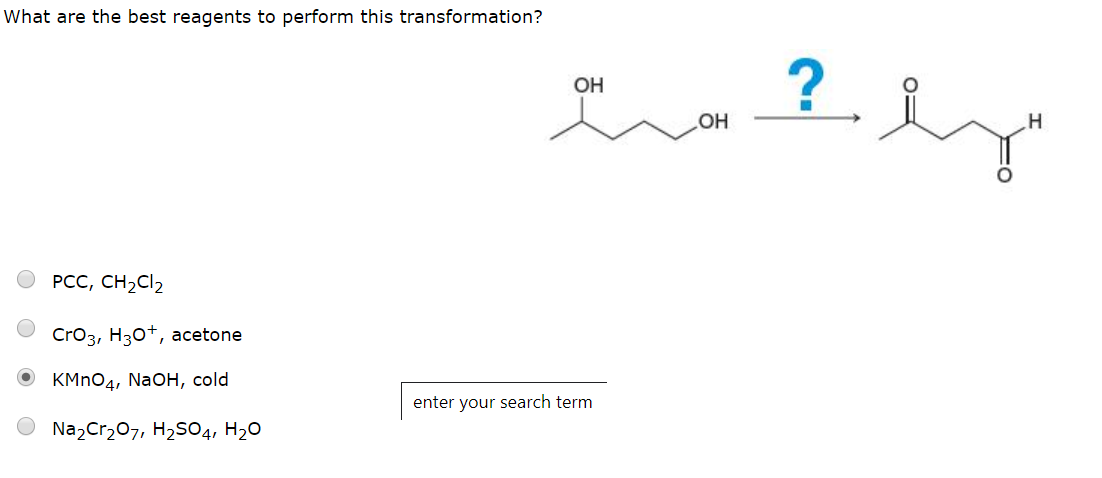
Transcribed Image Text:What are the best reagents to perform this transformation?
?
ОН
Он
н
РСС, CH2CI2
CrO3, H30, acetone
OKMnO4, Na OH, cold
enter your search term
Na2Cr207, H2SO4, H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
