What energy change is associated with the reaction to obtain one mole of H2 from one mole of water vapor? The balanced equation is 2 H20(g)2H2(g) + O2(g) and the relevant bond energies are: HH = 436 kJ/mol; H- O 467 kJ/mol; O - O 146 kJ/mol; O O = 498 kJ/mol.
What energy change is associated with the reaction to obtain one mole of H2 from one mole of water vapor? The balanced equation is 2 H20(g)2H2(g) + O2(g) and the relevant bond energies are: HH = 436 kJ/mol; H- O 467 kJ/mol; O - O 146 kJ/mol; O O = 498 kJ/mol.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 112AE: Compare your answers from parts a and b of Exercise 69 of Chapter 3 with H values calculated for...
Related questions
Question
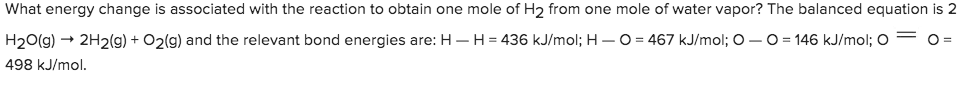
Transcribed Image Text:What energy change is associated with the reaction to obtain one mole of H2 from one mole of water vapor? The balanced equation is 2
H20(g)2H2(g) + O2(g) and the relevant bond energies are: HH = 436 kJ/mol; H- O
467 kJ/mol; O - O
146 kJ/mol; O
O =
498 kJ/mol.
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 8 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax